Text Solution
Verified by Experts
Topper's Solved these Questions
QUANTUM THEORY
CHHAYA PUBLICATION|Exercise CBSE Scanner|29 VideosQUANTUM THEORY
CHHAYA PUBLICATION|Exercise Entrance Corner (Integer answer type)|9 VideosOPTICAL INSTRUMENTS
CHHAYA PUBLICATION|Exercise CBSE SCANNER|8 VideosQUESTION PAPER OF NEET 2017
CHHAYA PUBLICATION|Exercise QUESTION PAPERS OF (NEET) (2017)|39 Videos
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-QUANTUM THEORY-Examination Archive with solutions
- Find the energy required by an electron to have its de Broglie wavelen...
Text Solution
|
- When green light is incident on a certain metal surface, electrons are...
Text Solution
|
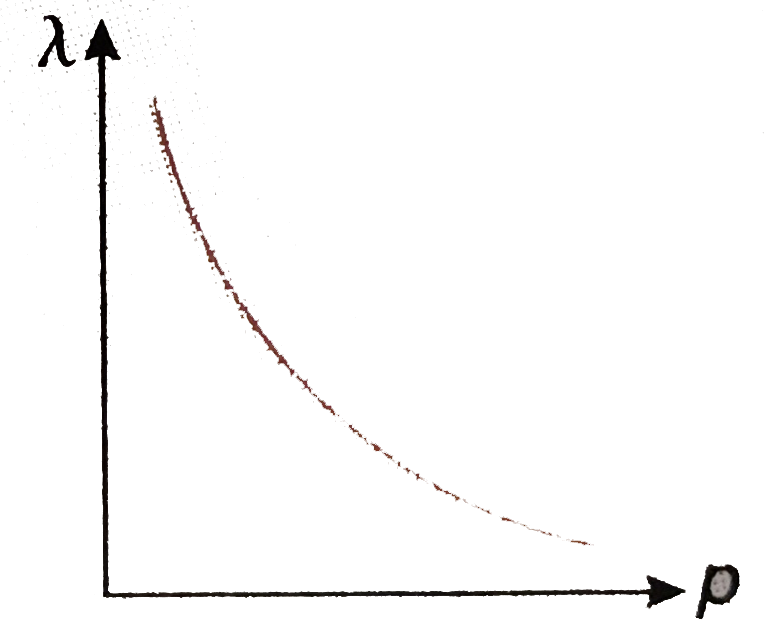
- Draw the curve showing the variation of de Broglie wavelength of a par...
Text Solution
|
- Mention the inference of Davission Garmer experiment
Text Solution
|
- Define stopping potential
Text Solution
|
- When light of wavelength lamda and 2 lamda are incident on a metal sur...
Text Solution
|
- The wavelength of de Broglie waves associated with a thermal neutron o...
Text Solution
|
- Find the correct statements about photoelectric effect.
Text Solution
|
- The de Broglie wavelength of an electron is the same as that 50 keV X-...
Text Solution
|
- The work function of metals is in the range of 2 eV to 5 eV. Find whic...
Text Solution
|
- Consider two particles of different masses. In which of the following ...
Text Solution
|
- The potential difference V required for accelerating an electron to ha...
Text Solution
|
- The work function of cesium is 2.27 eV. The cut-off voltage wich stops...
Text Solution
|
- The distance between a light source and photoelectric cell is d. If th...
Text Solution
|
- The de Broglie wavelength of an electron is 0.4 xx 10^(-10) m when its...
Text Solution
|
- When light of frequency v(1) is incident on a metal with work function...
Text Solution
|
- An electron accelerated through a potential of 10000 V from rest has a...
Text Solution
|
- Radiation of wavelength lamda is incident on a photocell. The fastest ...
Text Solution
|
- A particle A of mass m and initial velocity v collides with a particle...
Text Solution
|
- When the energy of the incident radiation is increased by 20%, the kin...
Text Solution
|