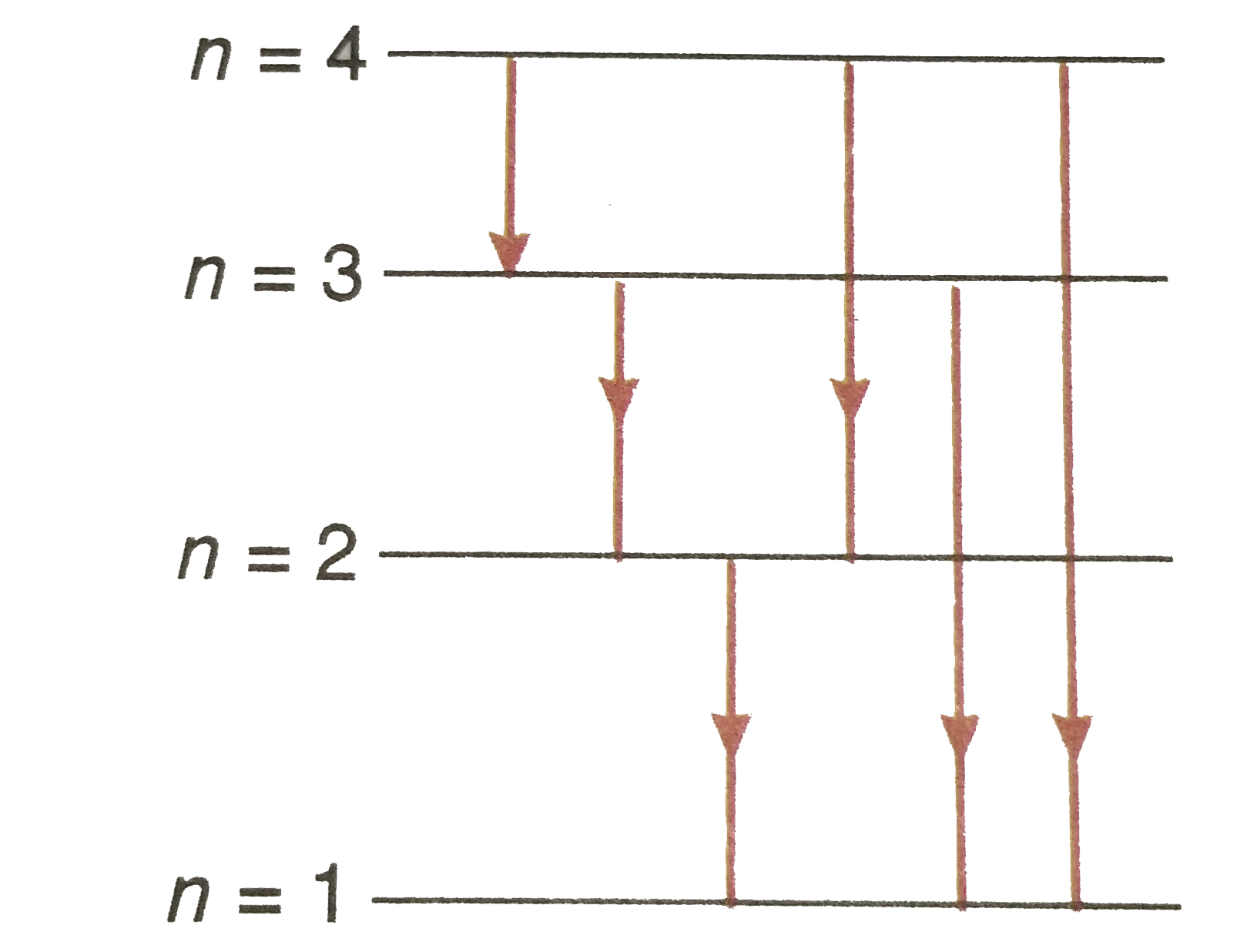
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CHHAYA PUBLICATION-ATOM-CBSE SCANNER
- Hydrogen atom in its ground state, is excited by means of monochromati...
Text Solution
|
- Using de Broglie's hypothesis , explain with the help of a suitable d...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV. what are the ...
Text Solution
|
- How are X-rays produced ?
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV . If an electron ...
Text Solution
|
- Using Bohr's postulates, derive the expression for the frequency of r...
Text Solution
|
- Using Rutherford model of atom , derive the expression for the total ...
Text Solution
|
- Using Bohr's postulates of the atomic model, derive the expression fo...
Text Solution
|
- Given the value of ground state energy of hydrogen atom as -13.6 eV. F...
Text Solution
|
- When is k(alpha) line in the emission spectrum of hydrogen atom obtain...
Text Solution
|
- Calculate the wavalength of radiation emitted when electron in a hydro...
Text Solution
|
- Define the distance of closest approach. An alpha-particle of kinetic ...
Text Solution
|
- Write two important of Rutheroford nuclear model of the atom. .
Text Solution
|
- Find out the wavelength of the electron orbiting in the ground state ...
Text Solution
|
- A 12.75 eV electron beam is used to excite a gaseous hydrogen atom at...
Text Solution
|
- The short wavelength limit for the Lyman series of the hydrogen spectr...
Text Solution
|
- The ground state energy of hydrogen atom is -13.6 eV . If an electron ...
Text Solution
|
- State Bohr's postulate to define stable orbits in hydrogen atom . How...
Text Solution
|
- A hydrogen atom initially in the ground state absorbs a photon which ...
Text Solution
|