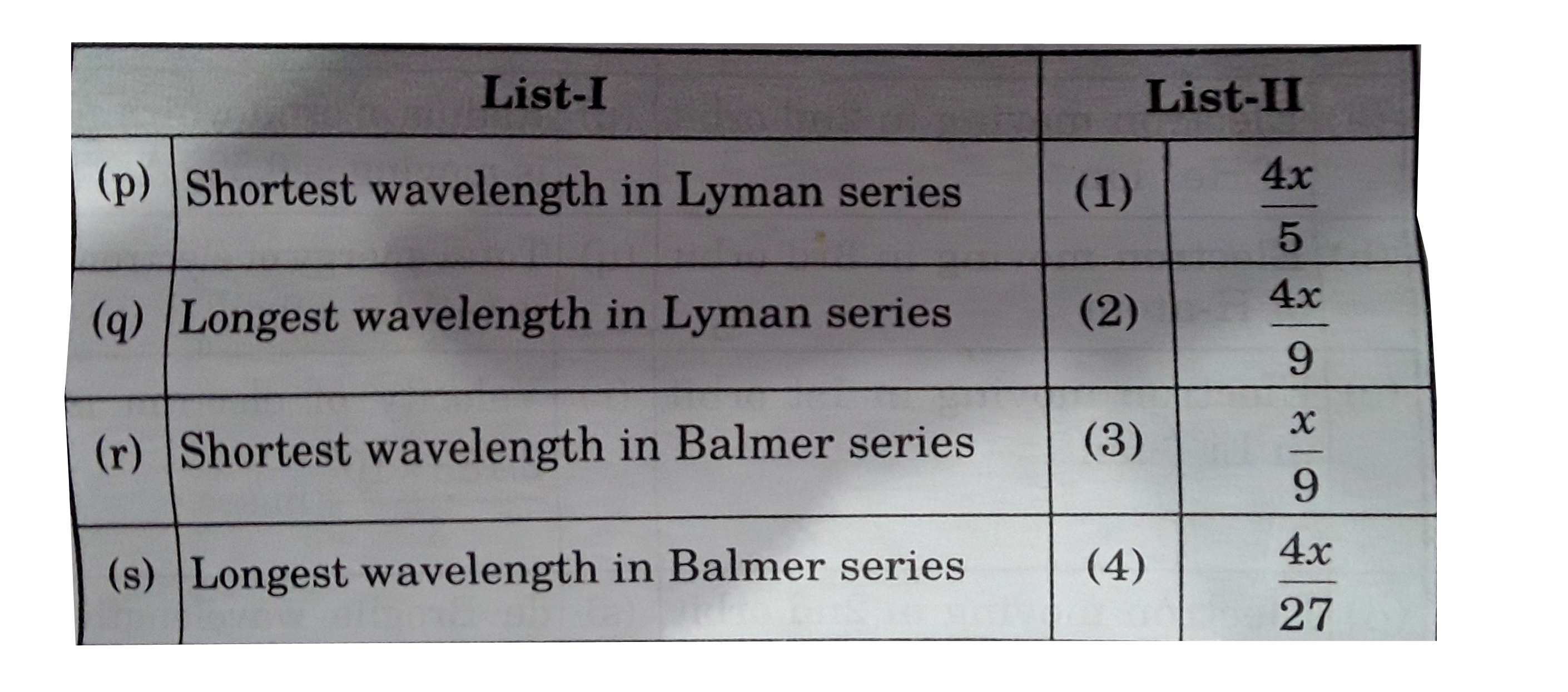
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ATOMIC STRUCTURE-Match the column type
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- E(n) = total energy , l(n) angular momentum K(n) = K.E., V(n) = P.E....
Text Solution
|
Text Solution
|
- Match the following columns
Text Solution
|
- Frequancy =f(1), Time period = T, Energy of n^(th) orbit = E(n), radiu...
Text Solution
|
- Match the following columns
Text Solution
|
- Given in hydrogen atom r(n),V(n),E,K(n) stand for radius, potential en...
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- r(n,z)= Radius of nth orbit of a single electron species having atomic...
Text Solution
|
- If the shortest wavelength of spectral line of H-atom in Lyman series ...
Text Solution
|
- In a scale of 10^(-18)m, match the particle with respect to their prob...
Text Solution
|
- Match the entries in column- I with the correctly related quantum numb...
Text Solution
|
- Psi(r) =k(1) e^(-r//k(2))(r^(2)-5k(3) r+6K(3)^(2)) For the above or...
Text Solution
|