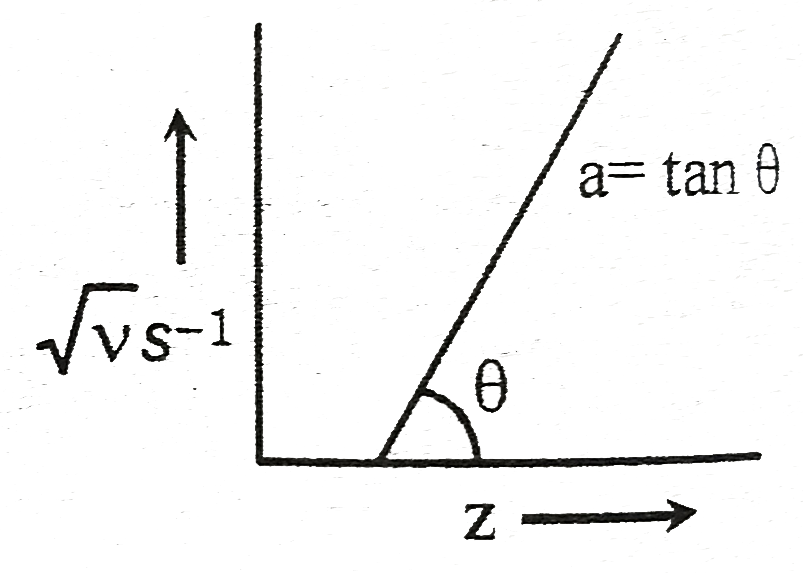A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-ATOMIC STRUCTURE-Exercise.3
- If the straight line is at an angle 45^(@) with b=1 calculate frequenc...
Text Solution
|
- The wavelenght of a certain line in the Paschen series in 1093.6 nm ...
Text Solution
|
- Calculate the Rydberg constant RH if He^+ ions are known to have the ...
Text Solution
|
- A photon having lambda = 854 Å cause the ionization of a nitrogen atom...
Text Solution
|
- H-atoms is exposed to electromagnetic radition of 1028Åand gives out ...
Text Solution
|
- The velocity of electron in a certain bohr orbit of hydrogen bears t...
Text Solution
|
- A doubly ionised lithium atom is hydrogen like with atomic number Z=3 ...
Text Solution
|
- Estimate the difference in energy between the first and second Bohr'...
Text Solution
|
- 1.8 g hydrogen atoms are excited to raditions . They study of spectra...
Text Solution
|
- If the average life time of an excited state of H atom is of order 10^...
Text Solution
|
- Calculate the binding energy per mole when threshold wavelength of pho...
Text Solution
|
- A potential difference of 20kV is applied across an X-ray tube. Find t...
Text Solution
|
- What is de-Broglie wavelength of a He-atom in a container at room temp...
Text Solution
|
- A portons is accelrated to one -tenth of the velocity of light . If it...
Text Solution
|
- To what effective potential a proton beam be subjected to give its pro...
Text Solution
|
- He atom can be excited to 1s^(1)2p^(1)"by"lambda=58.44 nm If lowest...
Text Solution
|
- The quantum yield for decomposition of HI id 0.2 .In an experiment ...
Text Solution
|
- Calculate the wavelength of the radiation that would cause photo disso...
Text Solution
|
- The dissociation energy of H(2) is 430.53 kJ mol^(-1), If H(2) is of d...
Text Solution
|
- X-rays emitted from a copper target and a molybdenum target are found ...
Text Solution
|
- An energy of 68eV is required to excite a hydrogen like atom from its...
Text Solution
|