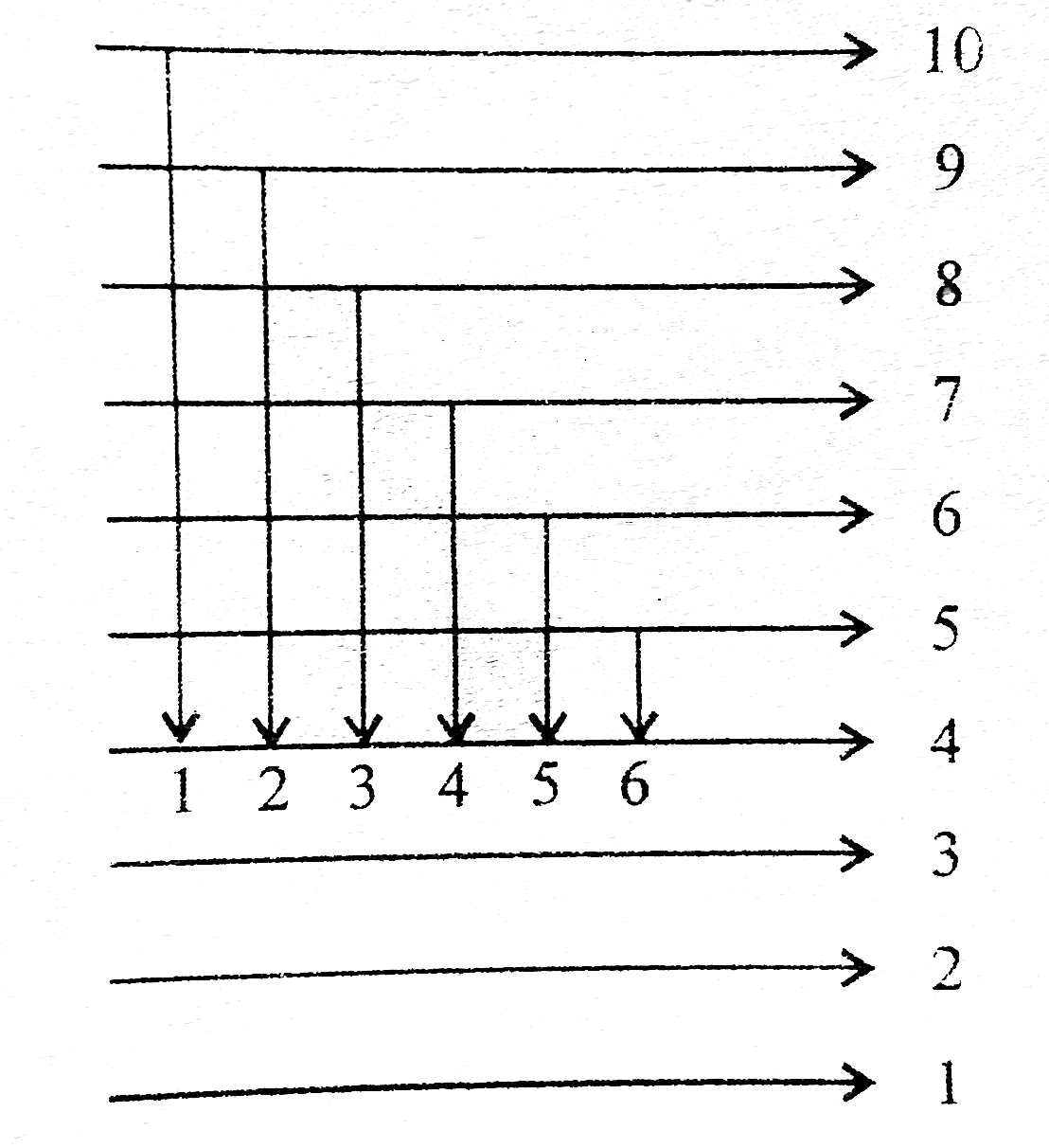A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-ATOMIC STRUCTURE-Exercise.1
- Three energy levels P,Q,R of a certain atom are such that E(P)ltE(Q)lt...
Text Solution
|
- The value of (n(2)+n(1)) and (n(2)^(2)-n(1)^(2)) for He^(+) ion in at...
Text Solution
|
- Number of possible sepectral lines which ,may br emitted in bracket s...
Text Solution
|
- The longest wavelength of He^(+) in paschen series is "m", then shor...
Text Solution
|
- When electron make transition to gound state, the largest wavelenght (...
Text Solution
|
- In sample of hydrogen atoms , electron jump from 10^(th) excited state...
Text Solution
|
- An electron in a hydrogen atom in its ground state absorbs energy equa...
Text Solution
|
- An electron, a proton and an alpha particle have kinetic energy of 16...
Text Solution
|
- For particles having same kinetic energy , the de Brogle wavelenght ...
Text Solution
|
- In a hydrogen atom , in transition of electron a photon of energy 2.55...
Text Solution
|
- An unknown particle having double charge as proton itself moves with w...
Text Solution
|
- If uncertainty in position and momentum are equal then uncertainty in ...
Text Solution
|
- If uncertainty in velocity is6.62xx10^(-2)m//sec . For a particle of m...
Text Solution
|
- What is uncerainty in location of photod of wavelenght 5000Å if wavel...
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The quatum numbers +(1)/(2) and -(1)/(2) for the electron spin represe...
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorbs a phot...
Text Solution
|
- The orbital angular momentum of an electron in 2s-orbital is
Text Solution
|
- Which quantum number is not related with Schrodinger equation :-
Text Solution
|
- The number of electrons present in Co^(2+) having azimuthal quantum ...
Text Solution
|