A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BANSAL-ATOMIC STRUCTURE-Exercise.1
- If uncertainty in position and momentum are equal then uncertainty in ...
Text Solution
|
- If uncertainty in velocity is6.62xx10^(-2)m//sec . For a particle of m...
Text Solution
|
- What is uncerainty in location of photod of wavelenght 5000Å if wavel...
Text Solution
|
- The number of nodal planes in a p(x) orbital is :
Text Solution
|
- The quatum numbers +(1)/(2) and -(1)/(2) for the electron spin represe...
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorbs a phot...
Text Solution
|
- The orbital angular momentum of an electron in 2s-orbital is
Text Solution
|
- Which quantum number is not related with Schrodinger equation :-
Text Solution
|
- The number of electrons present in Co^(2+) having azimuthal quantum ...
Text Solution
|
- Which of the following may represent the possbile quantum numbers for...
Text Solution
|
- Which wriiting the following electron cofiguration of Fe some rule hav...
Text Solution
|
- A compound of vanadium has a magnetic moment of 1.73BM. Work out th...
Text Solution
|
- Increasing order of magnetic monent among the following species is ...
Text Solution
|
- The Wave function (Psi) of 2s is given by: Psi(2s)=(1)/(2sqrt2pi)(1/...
Text Solution
|
- The wave function for 1s orbital of hydrogen atom is given by: Psi(1...
Text Solution
|
- The radial wave equyation for hydrogen of radial nodes from nucleus ar...
Text Solution
|
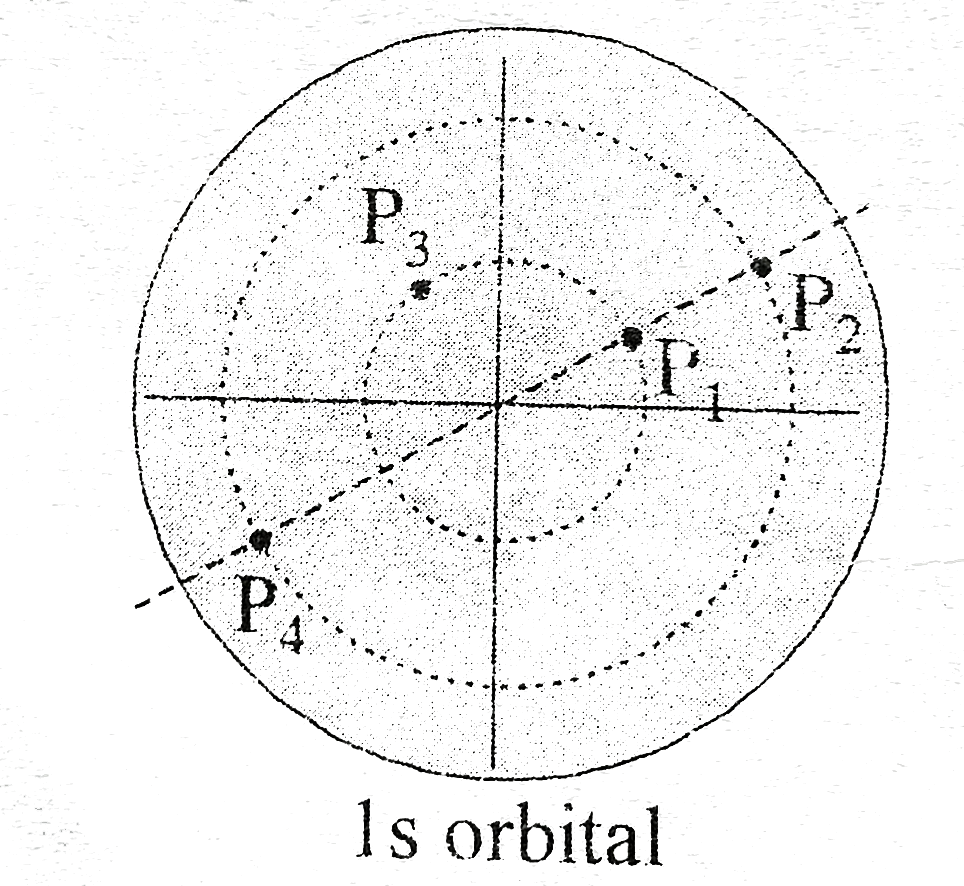
- The given diagram shows point P(1),P(2)P(3)&P(4) in 1s orbital. ...
Text Solution
|
- A 4pir^(2)Psi^(2)(r) vs" " r is ploted for H-orbital curve contains...
Text Solution
|
- For a unielectronic spiecie , compere the energy of the following orb...
Text Solution
|
- The radial propbabillity of finding an electron at spherical surface ...
Text Solution
|