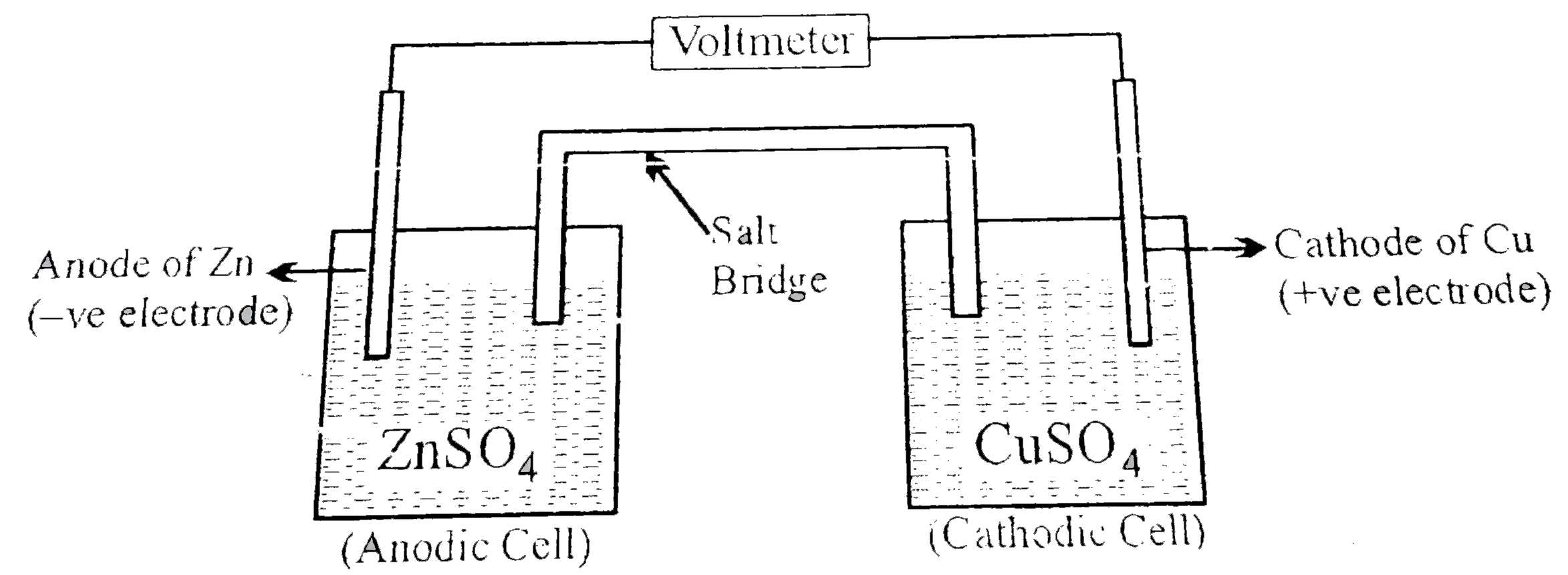
The most pupular electrochemical cell, daniel cell was originally developed by the English chemist john F daniel the general assembly of the cell is as given below An undergraduate student made a Daniel cell using `100cm^(3)` of `0.100MCuSO_(4)` ad `0.100M` `ZnSO_(4)` solution respectively. The two compartments are connected by suitable salt bridge.
[given `E_((Cu^(2+)//Cu))^(@)=0.34V,E_((Zn^(2+)//Zn))=-0.76V,(2.30RT)/(F)=0.06,log2=0.3]`
Q. Calculate the emf (in volt) of the above cell.