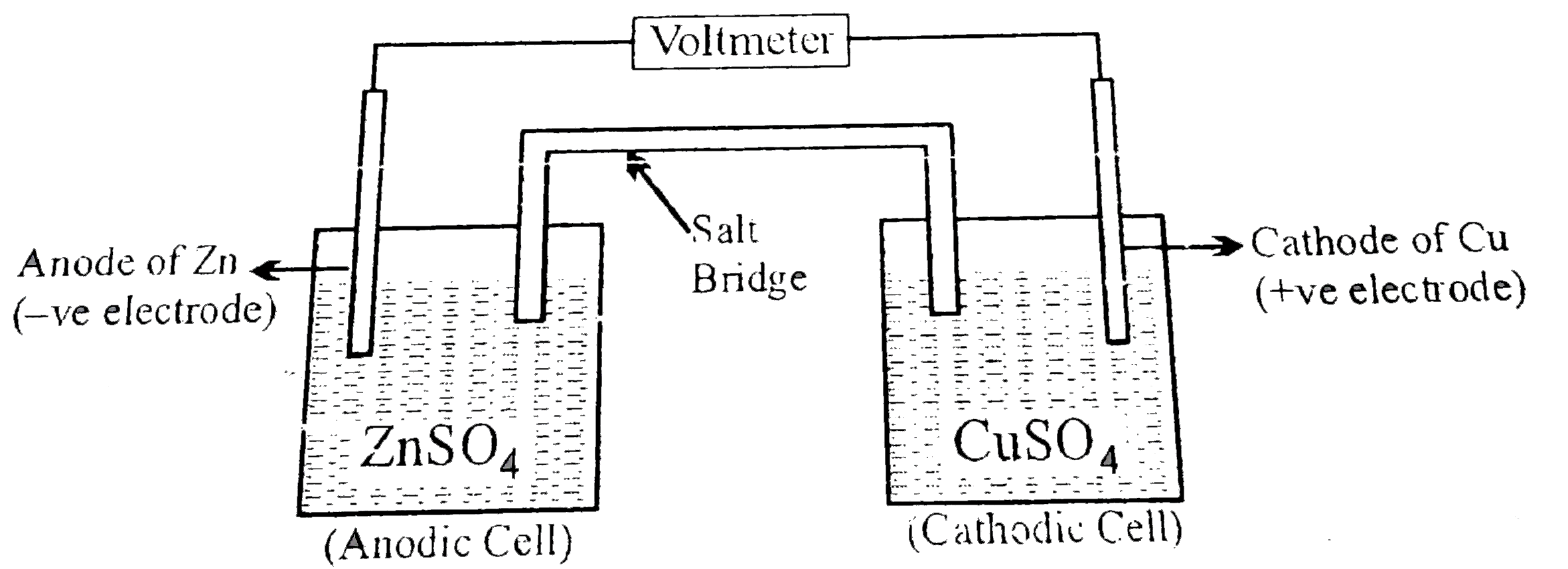
The most pupular electrochemical cell, daniel cell was originally developed by the English chemist john F daniel the general assembly of the cell is as given below An undergraduate student made a Daniel cell using `100cm^(3)` of `0.100MCuSO_(4)` ad `0.100M` `ZnSO_(4)` solution respectively. The two compartments are connected by suitable salt bridge.
[given `E_((Cu^(2+)//Cu))^(@)=0.34V,E_((Zn^(2+)//Zn))=-0.76V,(2.30RT)/(F)=0.06,log2=0.3]`
Q. A labmate of the student asked her for some solid `CuCl_(2)`. while she was lifting the bottle from a shelf, the lid of the bottle slipped and some amount of `CuCl_(2)` fell in the `CuSO_(4)` compartment at constant volume. She measured the emf of the cell again and found that it has increase by 9 mV. She used this data to calculate the amount of `CuCl_(2)` that had spilled in the compartment. Calculate the mass of `CuCl_(2)` that had spilled into the daniel cell? (molar mass of `CuCl_(2)=135(g)/(mol))`