Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-HYDROGEN AND ITS COMPOUNDS -Long Answer Questions
- Write an essay on the commericial preparation of dihydrogen. Give bala...
Text Solution
|
- Illustrate the chemistry of dihydrogen by its reaction with (i) N(2)
Text Solution
|
- Illustrate the chemistry of dihydrogen by its reaction with (b) Meta...
Text Solution
|
- Illustrate the chemistry of dihydrogen by its reaction with (c ) Org...
Text Solution
|
- Explain, with suitable examples, the following: (i) Electron-deficie...
Text Solution
|
- Explain, with suitable examples, the following: (ii) Electron - prec...
Text Solution
|
- Explain, with suitable examples, the following: (iii) Electron-rich ...
Text Solution
|
- Write in brief on (i) ionic hydrides
Text Solution
|
- Write in brief on (ii) interstitial hydrides.
Text Solution
|
- Explain any four of the chemical properties of water.
Text Solution
|
- Explain the terms hard water and soft water. Write a note on the (i)...
Text Solution
|
- Explain the terms hard water and soft water. Write a note on the (ii...
Text Solution
|
- Write the chemical reaction to justify that hydrogen peroxide can func...
Text Solution
|
- Complete and balance the following chemical reactions: (i) PbS(s) + ...
Text Solution
|
- Complete and balance the following chemical reactions: (ii) MnO(4)^(...
Text Solution
|
- Complete and balance the following chemical reactions: (iii) CaO(s) ...
Text Solution
|
- Complete and balance the following chemical reactions: (iv) Ca(3)N(2...
Text Solution
|
- Discuss, with relevant chemical equations, various methods of preparin...
Text Solution
|
- In how many ways can you express the strength of H(2)O(2)? Calculate t...
Text Solution
|
- Explain the structure of Hydrogen peroxide molecule.
Text Solution
|
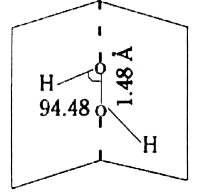 .
.