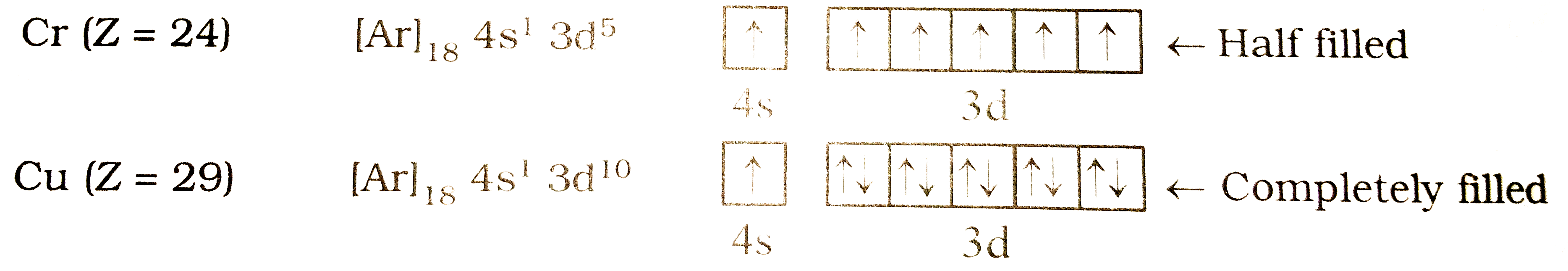
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise TEXTUAL LESSON PART ( ASKING QUESTIONS AND MAKING HYPOTHESIS)|2 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise TEXTUAL LESSON PART ( INFORMATION SKILLS AND PROJECTS)|1 VideosREFLECTION AND REFRACTION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|24 VideosSUMMATIVE ASSESSMENT
VGS PUBLICATION-BRILLIANT|Exercise SUMMATIVE ASSESSMENT|28 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STRUCTURE OF ATOM -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - IV 4 MARKS )
- Why there are exemptions in writing the electronic configurations of C...
Text Solution
|
- What are the postulates of Bohr's model of hydrogen atom?
Text Solution
|
- What are the limitations of Bohr's theory of hydrogen atom?
Text Solution
|
- Draw Moeller chart of Ctlling order of atomic orbitals.
Text Solution
|
- Draw a diagram showing the increasing value of (n + l) of orbitals.
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Based on the information given in the table , answer the question give...
Text Solution
|
- Observe the information provided in the table about quantum numbers. T...
Text Solution
|
- Observe the information provided in the table about quantum numbers. T...
Text Solution
|
- Observe the information provided in the table about quantum numbers. T...
Text Solution
|
- Observe the information provided in the table about quantum numbers. T...
Text Solution
|
- Explain Bohr’s model of hydrogen atom and its limitations.
Text Solution
|
- Explain Bohr- Sommerfeld model of an atom. What is the merit of this m...
Text Solution
|
- Do the electrons follow defined paths around the nucleus
Text Solution
|
- What are the main features of quantum mechanical model of an atom?
Text Solution
|
- Prepare some questions to ask and make your friend understand the conc...
Text Solution
|
- Anitha argued that the 3d orbital is filled first, but not 4s orbital,...
Text Solution
|
- In an atom the number of electrons in N - Shell is equal to the number...
Text Solution
|
- In an atom the number of electrons in N - Shell is equal to the number...
Text Solution
|