Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - III 2 MARKS QUESTIONS )|38 VideosREFLECTION AND REFRACTION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|24 VideosSUMMATIVE ASSESSMENT
VGS PUBLICATION-BRILLIANT|Exercise SUMMATIVE ASSESSMENT|28 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STRUCTURE OF ATOM -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - IV 4 MARKS )
- Explain Bohr’s model of hydrogen atom and its limitations.
Text Solution
|
- Explain Bohr- Sommerfeld model of an atom. What is the merit of this m...
Text Solution
|
- Do the electrons follow defined paths around the nucleus
Text Solution
|
- What are the main features of quantum mechanical model of an atom?
Text Solution
|
- Prepare some questions to ask and make your friend understand the conc...
Text Solution
|
- Anitha argued that the 3d orbital is filled first, but not 4s orbital,...
Text Solution
|
- In an atom the number of electrons in N - Shell is equal to the number...
Text Solution
|
- In an atom the number of electrons in N - Shell is equal to the number...
Text Solution
|
- In an atom the number of electrons in N - Shell is equal to the number...
Text Solution
|
- In an atom the number of electrons in N - Shell is equal to the number...
Text Solution
|
- 1) How man.y orbitals are there in p-subshell ? 2) How many electro...
Text Solution
|
- a) 1s^(2) 2s^(2) 2p^(3) implies (b) 1s^(2) 2s^(2) 2p^(6) 3s^(2...
Text Solution
|
- a) 1s^(2) 2s^(2) 2p^(3) implies (b) 1s^(2) 2s^(2) 2p^(6) 3s^(2...
Text Solution
|
- a) 1s^(2) 2s^(2) 2p^(3) implies (b) 1s^(2) 2s^(2) 2p^(6) 3s^(2...
Text Solution
|
- a) 1s^(2) 2s^(2) 2p^(3) implies (b) 1s^(2) 2s^(2) 2p^(6) 3s^(2...
Text Solution
|
- Explain electromagnetic spectrum. Draw its diagram.
Text Solution
|
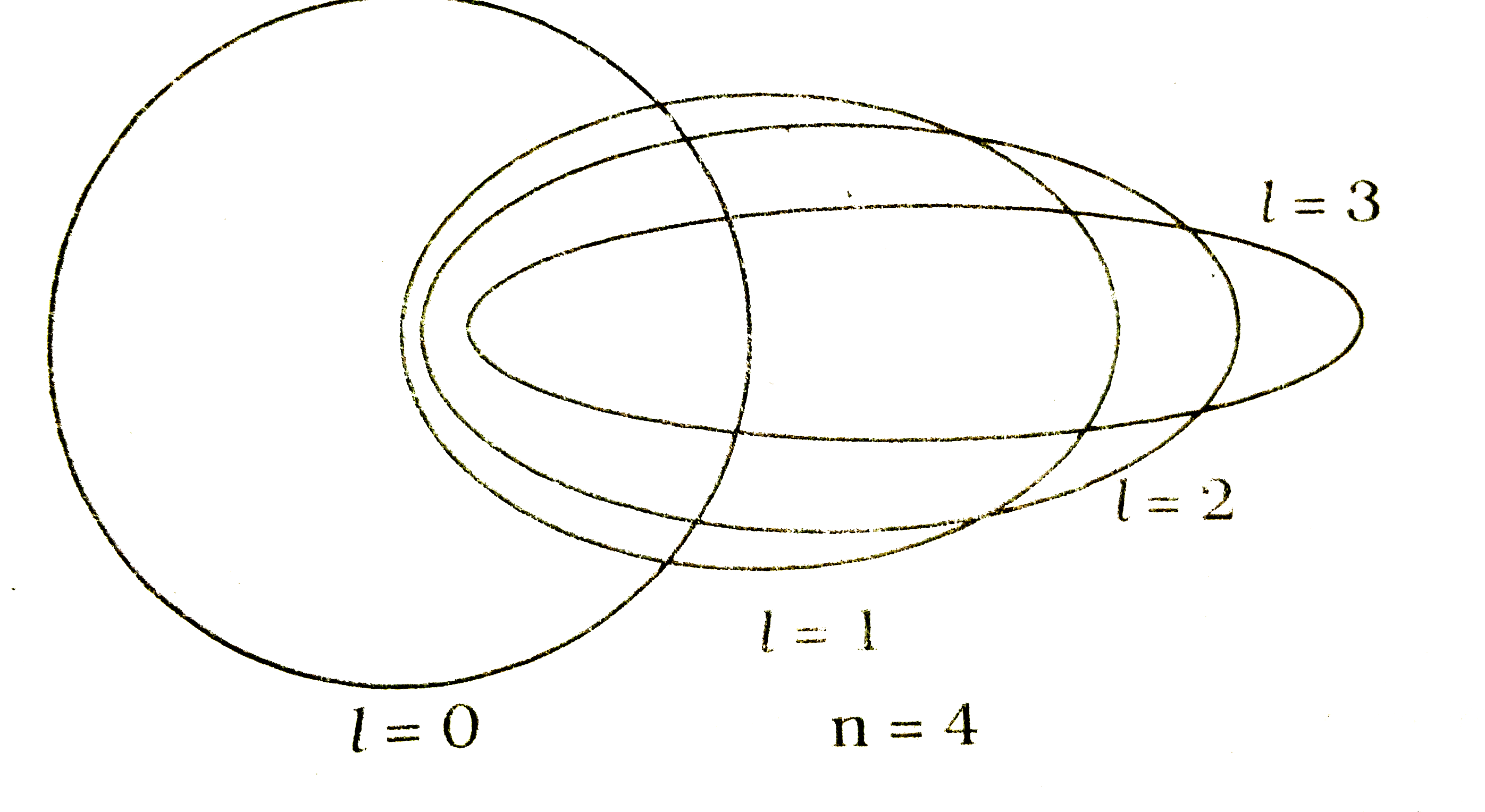
- Draw the electronic orbits for the main quantum number n = 4 by Bohr-S...
Text Solution
|
- Draw the shapes of s, p and d orbitals.
Text Solution
|
- How do you appreciate Niels Bohr for his contributions to under standi...
Text Solution
|
- How do you appreciate the scientists Aufbau and Hund?
Text Solution
|