Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (APPRECIATION AND AESTHETIC SENSE , VALUES )|5 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY )|4 VideosCLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (ASKING QUESTIONS AND MAKING HYPOTHESIS )|3 VideosCHEMICAL REACTIONS AND EQUATIONS
VGS PUBLICATION-BRILLIANT|Exercise OBJECTIVE TYPE QUESTIONS|80 VideosCLASSIFICATION OF ELEMENTS-THE PERIODIC TABLES
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|351 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CLASSIFICATION OF ELEMENTS -THE PERIODIC TABLE -CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION (SECTION -IV) (INFORMATION SKILLS AND PROJECTS )
- ""(5 ) ""(6 ) C ,""(7 ) N , ""(8 ) O ,""(9 ) Fe .""(10 ) Ne ar...
Text Solution
|
- ""(5 ) ""(6 ) C ,""(7 ) N , ""(8 ) O ,""(9 ) Fe .""(10 ) Ne ar...
Text Solution
|
- ""(5 )B ""(6 ) C ,""(7 ) N , ""(8 ) O ,""(9 ) F .""(10 ) Ne ar...
Text Solution
|
- Group : Be - Mg -Ca -Sr -Ba -Ra Period : Na -Mg -Al - Si - S -C...
Text Solution
|
- Write the elements in the ascending order of their atomic radii
Text Solution
|
- Some elements belonging to second period of periodic table , an...
Text Solution
|
- Some elements belonging to second period of periodic table , an...
Text Solution
|
- Some elements belonging to second period of periodic table , an...
Text Solution
|
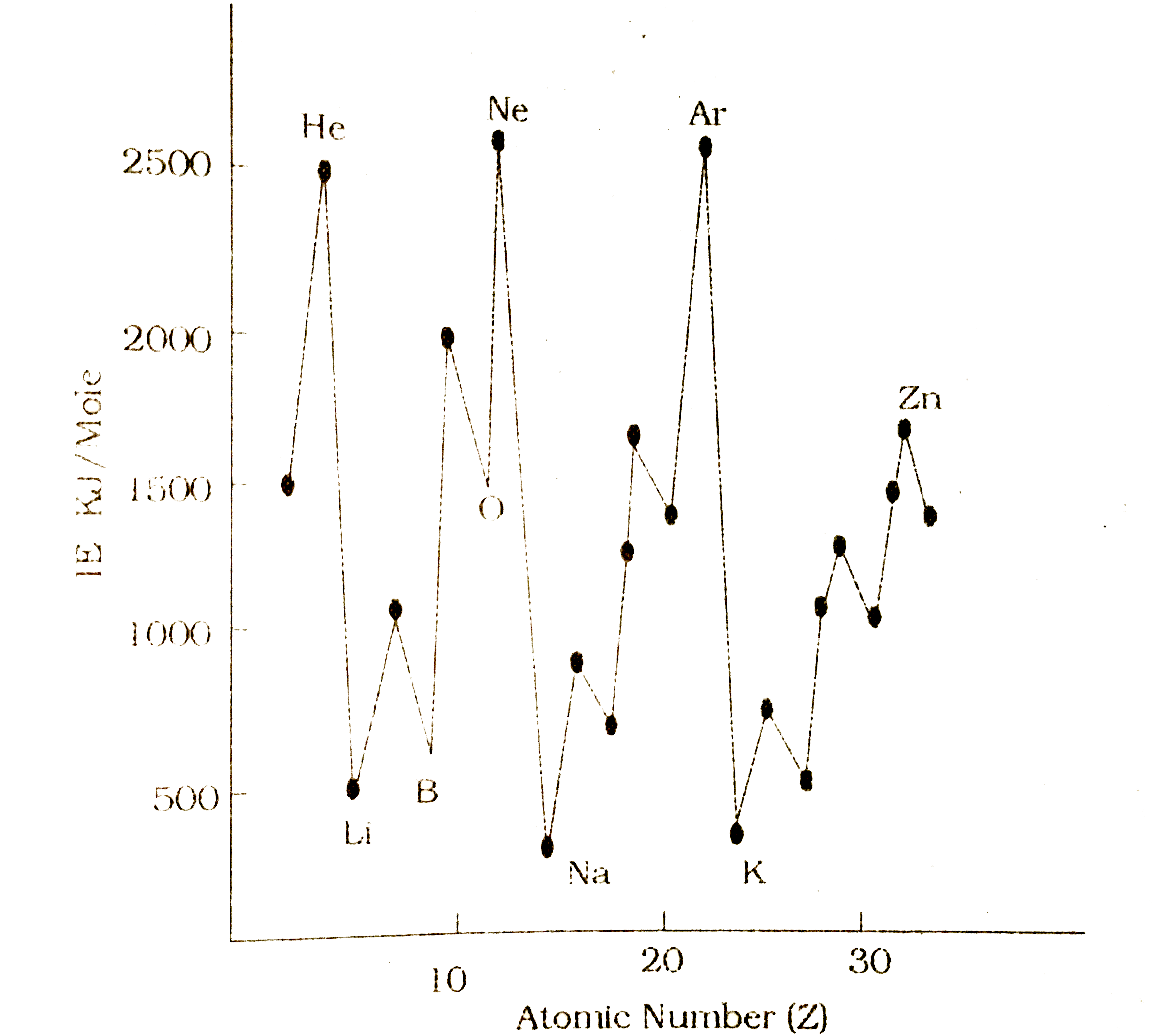
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- Ionization Fotential curve is the group of atomic number versus...
Text Solution
|
- The electrons configuration of atom A is 2,8,6 What is the a...
Text Solution
|
- The electrons configuration of atom A is 2,8,6 stable whethe...
Text Solution
|
- The electrons configuration of atom A is 2,8,6 Which of the ...
Text Solution
|
- The electrons configuration of atom A is 2,8,6 How the eleme...
Text Solution
|
- Refer the above part periodic table and answer the following ...
Text Solution
|
- Refer the above part periodic table and answer the following ...
Text Solution
|
- Refer the above part periodic table and answer the following ...
Text Solution
|
- Refer the above part periodic table and answer the following ...
Text Solution
|