A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-ACIDS,BASES AND SALTS-7 . Carbon and its Compounds
- Vinegar is a solution of
Text Solution
|
- Pentane has the molecular formula C(5) H(12 . It has
Text Solution
|
- Which among the following are unsturated hydrocarbons ? a) H(3) C ...
Text Solution
|
- Which is correct IUPAC name of the following compound. CH(3)-overs...
Text Solution
|
- Observe the following table carefully . {:("Test tube","Hard water"...
Text Solution
|
- Study the reaction given below . underset("Acetic acid")(CH(3)-COOH...
Text Solution
|
- A molecule of ammonia (NH(3)) has
Text Solution
|
- Mineral acids are stronger acids than cardoxylic acids because
Text Solution
|
- The reaction of an alcohol with carboxylic acid is called
Text Solution
|
- In the presence of concentrated sulphuric acid acetic acid reacts wit...
Text Solution
|
- Which of the following has shortest carbon-carbon bond length ?
Text Solution
|
- Which of the following are isomers ?
Text Solution
|
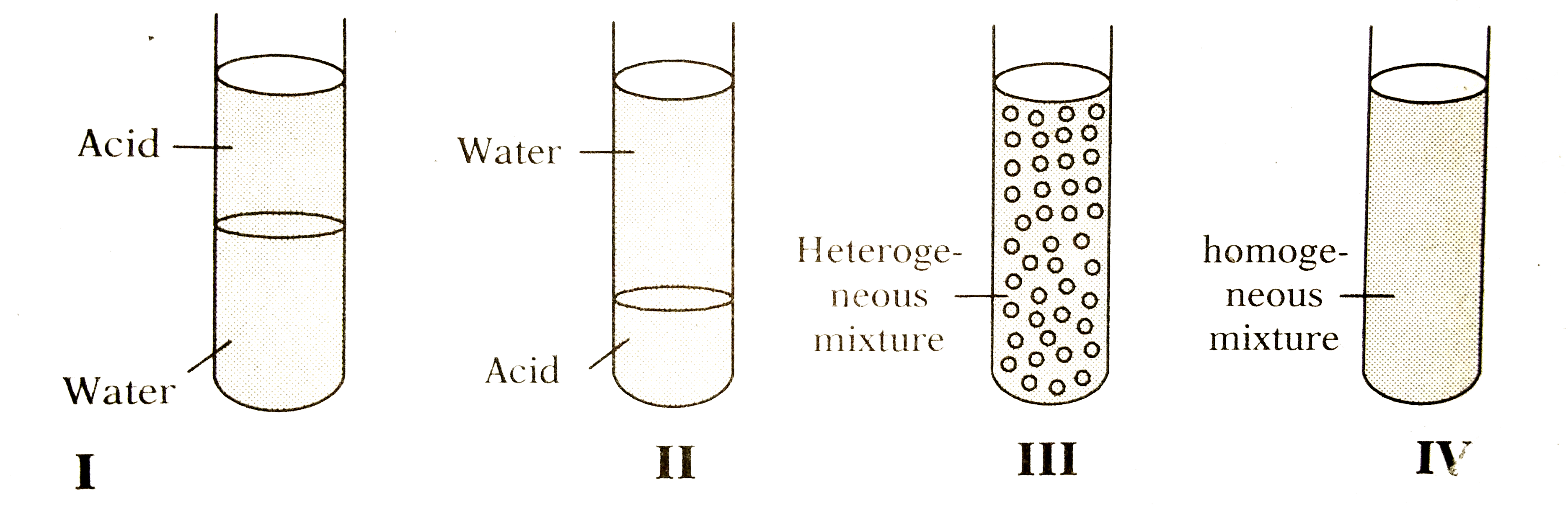
- Amount of 5 ml each of acetic acid and water are mixed together and sh...
Text Solution
|
- Which of the following statements are usually correct for carbon compo...
Text Solution
|
- The structures of four organic compounds are shown below . Which c...
Text Solution
|
- The diagram shows the structure of ethanoic acid . H-underset(H)und...
Text Solution
|
- Which of the following are correct structural isomers of butane ?
Text Solution
|