A small spherical monoatomic ideal gas bubble `(gamma=5//3)` is trapped inside a liquid of density `rho` (see figure). Assume that the bubble does not exchange any heat with the liquid. The bubble contains n moles of gas. The temperature of the gas when the bubble is at the bottom is `T_0`, the height of the liquid is H and the atmospheric pressure `P_0` (Neglect surface tension).
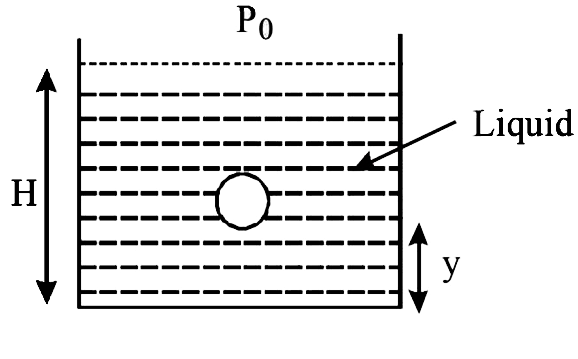
When the gas bubble is at a height y from the bottom, its temperature is-