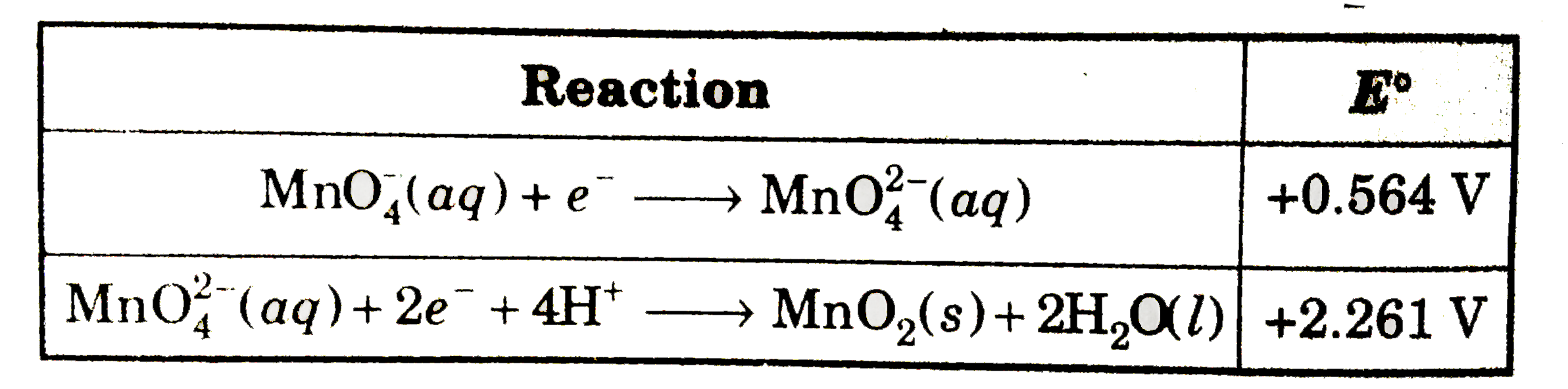A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Batteries|10 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Thermodynamics in Electrochemistry|22 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Galvanic Cell and Salt Bridge|85 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Latimer Diagram, concentration cells
- For which half-reaction will a 0.1 unit increase in pH cause the great...
Text Solution
|
- In a concentration cell, Zn|Zn^(2+) (0.1M) || Zn^(2+) (0.15M) | Zn, as...
Text Solution
|
- Use the given standard reduction potentials to determine the reduction...
Text Solution
|
- The standard electrode potential for the reactions, Ag^(+)(aq)+e^(-)...
Text Solution
|
- The standard electrode potentials, E^(@) of Fe^(3+)//Fe^(2+) and Fe^(2...
Text Solution
|
- The emf of a cell corresponding to the following reaction is 0.0199 V ...
Text Solution
|
- Ni(s) + Cu^(2+)(aq) rightarrow Ni^(2+)(aq) + Cu(s) The voltaic cell ...
Text Solution
|
- For which of these oxidation/reduction pairs will the reduction potent...
Text Solution
|
- A variable, opposite external potential(E(ext) is applied to the ce...
Text Solution
|
- For the galvanic cell: Ag(s) | AgBr(aq) | Br^(-)(aq) || Cl^(-)(aq)| ...
Text Solution
|
- At 298 K, the standard reduction potential are: 1.33 V for Cr(2)O(7)^(...
Text Solution
|
- Which of the given cells are concentration cells? (P) Pt|H(2)(p(1)ba...
Text Solution
|
- In given galvanic cell, Pt|H-(2)(g) | NaCl(aq)|| AgNO(3)(aq)| Ag o...
Text Solution
|
- At 298 K, the standard reduction potentials are 1.51 V for MnO(4)^(-) ...
Text Solution
|
- Latimer diagram for Cu in an acidic solution is: Cu^(+2) overset(+0....
Text Solution
|
- The reduction of O(2) to H(2)O in acidic solution has a standard reduc...
Text Solution
|
- Pt(s), H(2)(g) |HCl(C(1))||HCl(C(2)|H(2),Pt(s) The emf of cell is at...
Text Solution
|
- Given the two standard reduction potentials below, what is the K(sp) o...
Text Solution
|
- An increase in pH will promote which of the reactions below? (P) 2Mn...
Text Solution
|
- Cu^(2+)(aq) + Zn(s) rightarrow Cu(s) + Zn^(2+)(aq), E^(@) = 1.1V Zn^...
Text Solution
|