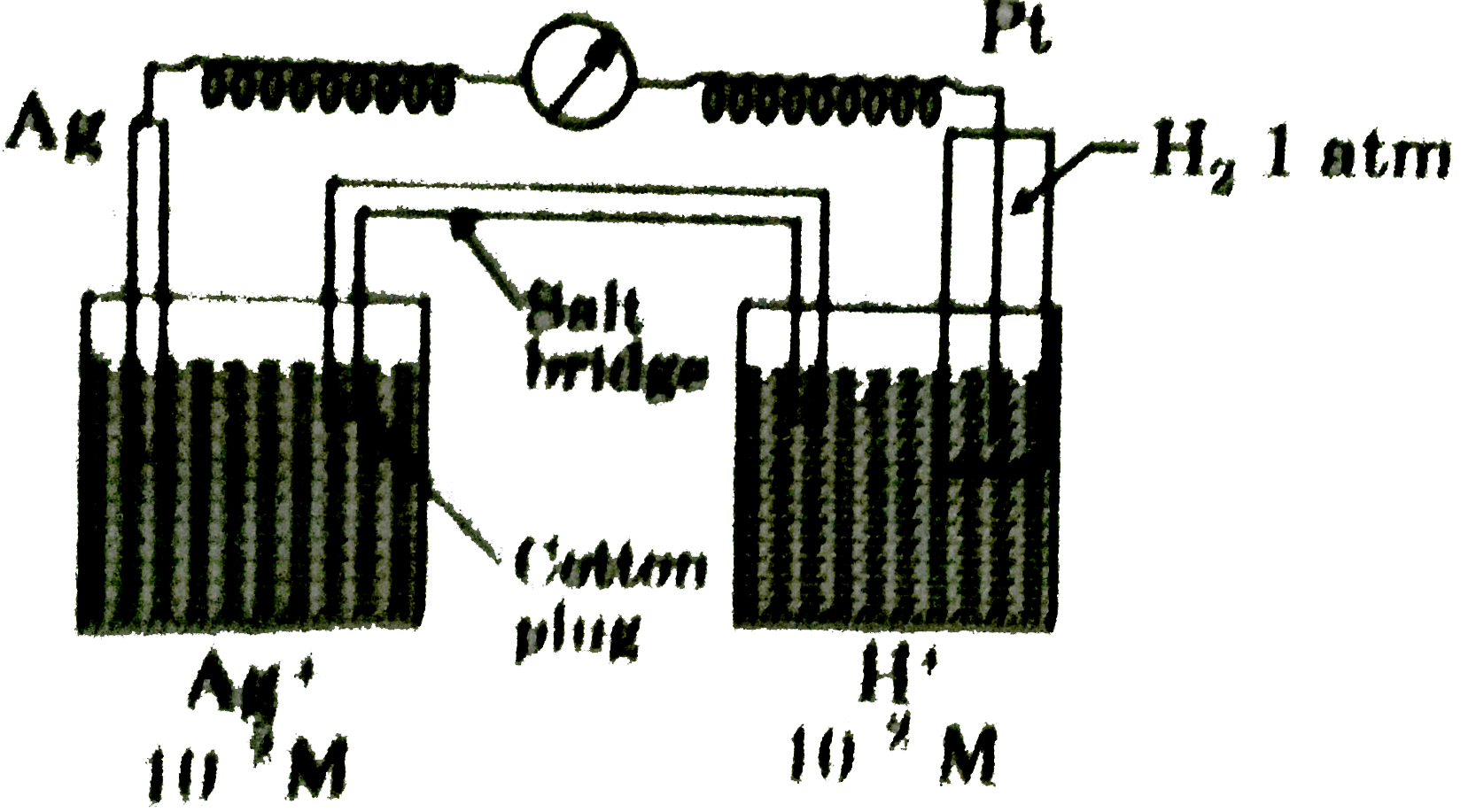
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Batteries|10 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Thermodynamics in Electrochemistry|22 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Galvanic Cell and Salt Bridge|85 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Latimer Diagram, concentration cells
- Given the reactions and data below, what is the K value for this elect...
Text Solution
|
- A voltaic cell is constructed with the overall reaction : Sn^(2+)(aq...
Text Solution
|
- Which change occurs as the chemical reaction takes place in the standa...
Text Solution
|
- Calculate the cell potential, E, for a silver-silver chloride electrod...
Text Solution
|
- Consider a voltaic cell in which the reaction below occurs in two half...
Text Solution
|
- 2Ag^(+)(aq) + Cu(s) rightarrow Cu^(2+)(aq) + 2Ag(s) The standard pot...
Text Solution
|
- Zn(s) | zn^(2+)(aq) || H(+)(aq)|H(2)(g) E^9@) = 0.76V What must be t...
Text Solution
|
- The voltage for the cell Fe | Fe^(2+)(0.0010 M) || Cu^(2+)(0.10 M) | C...
Text Solution
|
- For the cell Zn(s) + 2H^(+)(aq) rightarow Zn^(2+)(aq) + H(2)(g), E^(...
Text Solution
|
- 2Ga(s) + 6H^(+)(aq) rightarrow 2Ga^(3+)(aq) + 3H(2)(g) , The potenti...
Text Solution
|
- The following cell Al(s) | Al^(3+(aq, 0.001 M) || Cu^(2+)(aq, 0.10 M...
Text Solution
|
- For the voltaic cell respresents below Ni(s) | Ni^(2+)(aq) || Ag^(+)...
Text Solution
|
- Which of the following is correct about the electrochemical cell repre...
Text Solution
|
- For the voltaic cel based on this reaction: 2Ag^(+)(aq) + Cu rightar...
Text Solution
|
- What is the [Fe^(2+)] in a cell at 25^(@)C for which E = -0.458V with ...
Text Solution
|
- Sn(s) | Sn^(2+)(aq) || Cu^(2+)(aq) | Cu(s) For the voltaic cell repr...
Text Solution
|
- Calculate emf of given cell at 25^(@)C: [Given: E(Ag^(+)//Ag)^(@)...
Text Solution
|
- The standard reduction potential for H^(+)(aq) is 0.00v. What is the r...
Text Solution
|
- Under what conditions is the Nernst equation used to calcualte cell po...
Text Solution
|
- Which is a consistent set of values for a specific redox reaction carr...
Text Solution
|