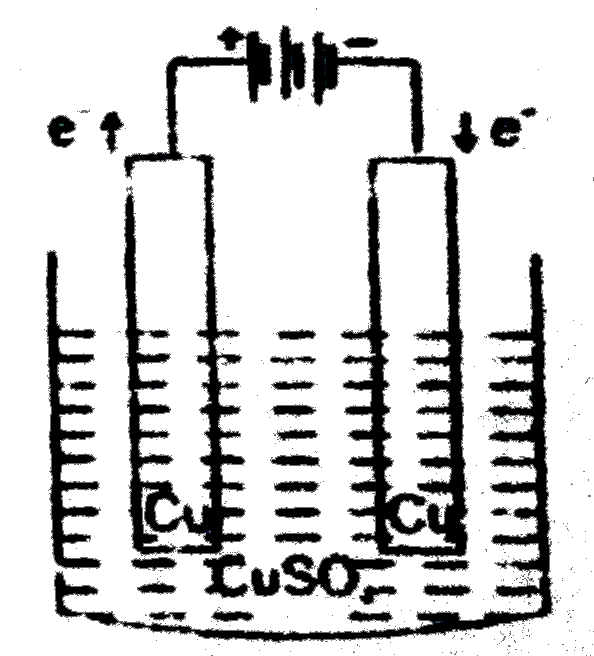A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Conductance and Kohlrausch s law|40 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Reasoning type|28 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Thermodynamics in Electrochemistry|22 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Faraday s Laws and Electrolysis
- In the electrolysis of an aqueous nickel (II) sulphate solution, the p...
Text Solution
|
- By the electrolysis of aqueous solution of CuSO(4), the products obtai...
Text Solution
|
- In the given figure the electrolytic cell contains 1L of an aqueous 1M...
Text Solution
|
- Aqueous solution of Na(2)SO(4) containing a small amount of HPh is ele...
Text Solution
|
- What happens to a certain during the electrolysis of a molten salt? Th...
Text Solution
|
- The passage of a constant current through a solution of dilute H(2)SO(...
Text Solution
|
- The current of 5A deposited 1.517 g of Pt in 10 min from a solution of...
Text Solution
|
- For the electrolytic production of NaClO(4) from NaClO(3) as per the f...
Text Solution
|
- 1.0 M aqueous solutions of AgNO(3), Cu(NO(3))(2) and Au(NO(3))(3) are ...
Text Solution
|
- Number of electrons lost dring electrolysis of 0.355g of Cl^(c-) is (N...
Text Solution
|
- Electrolysis of a solution of MnSO(4) in aqueous sulphuric acid is a m...
Text Solution
|
- An alloy of lead (Valency = 2) and thallium (valency = 1) containing 6...
Text Solution
|
- During the preparation of H(2)S(2)O(8) (per disulphuric acid) O(2) gas...
Text Solution
|
- A current of 9.95 amp following for 10 minutes, deposits 3 gm of a met...
Text Solution
|
- Four moles of electrons were transferred from anode to cathode in an e...
Text Solution
|
- Electrolytic reduction of 6.15 g of nitrobenzene using a current effic...
Text Solution
|
- A certain current liberates 0.5g of hydrogen in 2 hours. How many gra...
Text Solution
|
- All of the following affect the number of "mole"s of metal deposited d...
Text Solution
|
- What is the amount of chlorine evoled when 2 amperes of current is pas...
Text Solution
|
- A spoon to be electroplated with gold should be placed at:
Text Solution
|