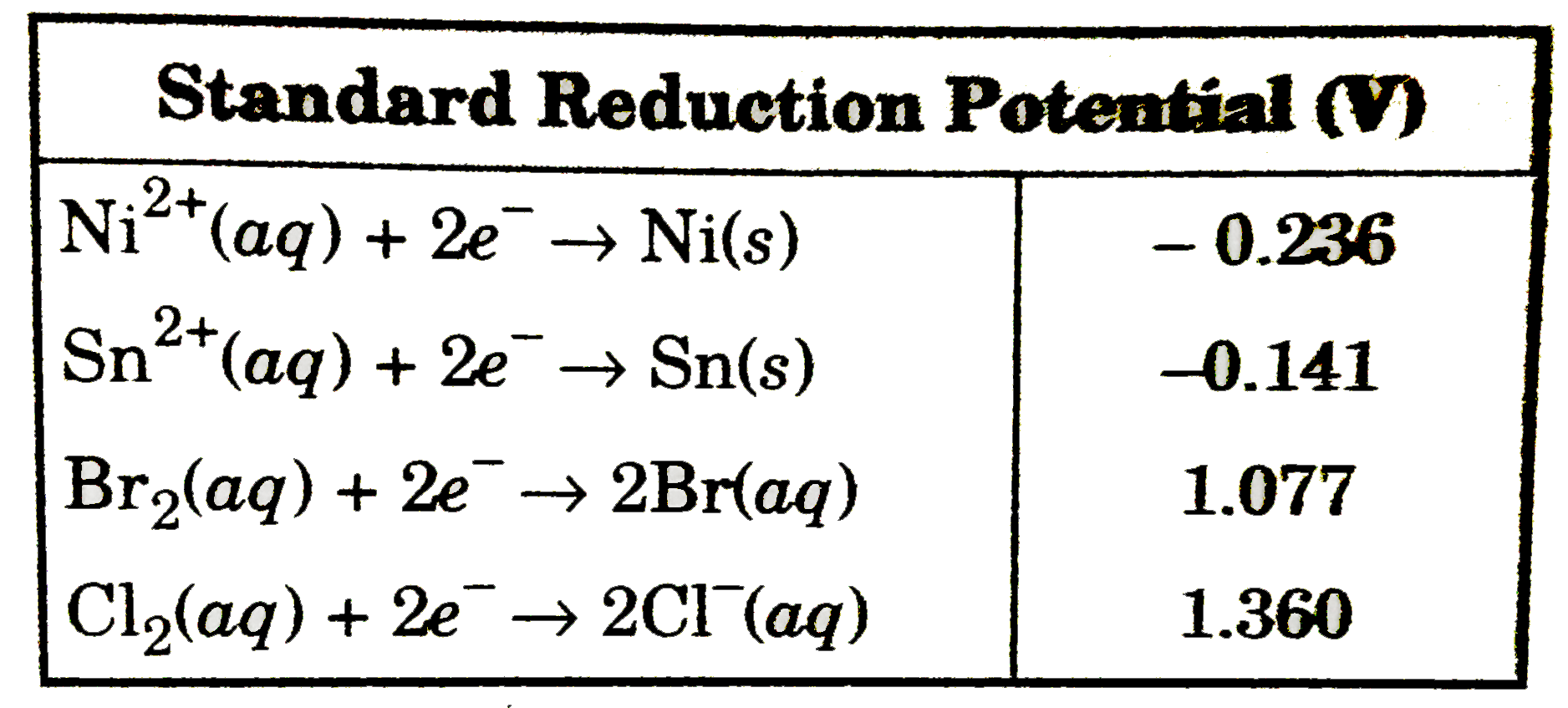
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTY
GRB PUBLICATION|Exercise Conductance and Kohlrausch s law|40 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Reasoning type|28 VideosELECTROCHEMISTY
GRB PUBLICATION|Exercise Thermodynamics in Electrochemistry|22 VideosD-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective Type|18 VideosENVIRONMENTAL CHEMISTRY
GRB PUBLICATION|Exercise Straight objective type|40 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Faraday s Laws and Electrolysis
- If aq. CuCl(2) solution is electrolysed by using Cu electrodes then pr...
Text Solution
|
- An electrolysis cell is operated for 3000 a using a current of 1.50A. ...
Text Solution
|
- Aluminium is produced commercially by the electrolysis of Al(2)O(3). H...
Text Solution
|
- How many liters of chlorine gas, Cl(2), measured at 0^(@)C and 1 atm a...
Text Solution
|
- For how many seconds must a current of 5.00 A flow in order to deposit...
Text Solution
|
- It takes 126.5 minutes using a current of 5.15 A to deposit all of the...
Text Solution
|
- A current of 0.0965 ampere is passed for 1000 seconds through 50mL of ...
Text Solution
|
- When a current of 0.25 ampere is passed through excess of molten MCl(x...
Text Solution
|
- In an electrolytic cell the electrode at which the electrons enter the...
Text Solution
|
- A solution containing equimolar amounts of NiCl(2) and SnBr(2) is elec...
Text Solution
|
- A current of 2.0A is used to plate Ni(s) from 500mL of a 1.0M Ni^(2+)(...
Text Solution
|
- What occurs when an aqueous solution of Na(2)SO(4) containing several ...
Text Solution
|
- A current of 12A is used to plate nickel from a Ni(NO(3))(2) solution....
Text Solution
|
- A 3.00amp current is used to electrolyze the molten chlorides, CaCl(2)...
Text Solution
|
- The deposition of 1.0g of which element from its molten chloride requi...
Text Solution
|
- Chromium metal can be produced by the electrolysis of molten CrO(3). W...
Text Solution
|
- Which reaction occurs at the cathode during electrolysis of an aqueous...
Text Solution
|
- When an aqueous solution of potassium fluoride is electrolyzed, which ...
Text Solution
|
- Given the standard reduction potentials. {:(O(2)+4H^(+)+4e^(-)rarr2H...
Text Solution
|
- In a battery with a zinc anode, what is the 250mA is drawn for 12.0 mi...
Text Solution
|