Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-ELECTROCHEMISTY-Match the column type
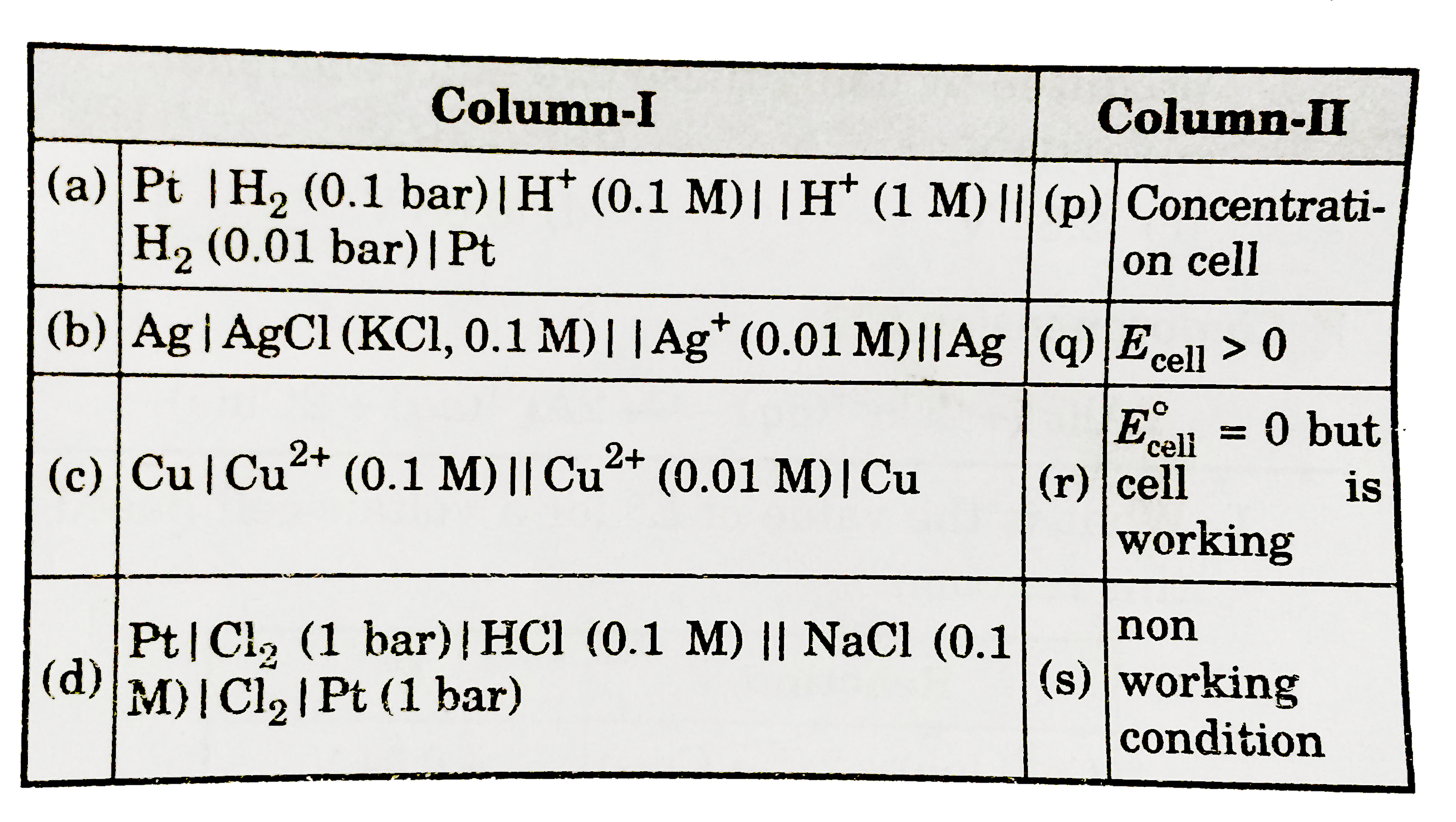
- Mathc the following columns :
Text Solution
|
- Match matrix (E(Ag^(+)//Ag)^(@)=0.8K(sp)(AgCl)=10^(-10))
Text Solution
|
Text Solution
|
- Match the following columns:
Text Solution
|
- {:(Column-I,Column-II),((A)"Conductance",(p)Cm^(-1)),((B)"Specific con...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Match the terms given in Column I with the items given in Column II. (...
Text Solution
|
- Match the items of Column I and Column II on the basis of data given b...
Text Solution
|
Text Solution
|
- If AgCl hArr Ag^(+) + Cl^(-) K(eq) = 10^(-10) AgBr hArr Ag6(+) + Br...
Text Solution
|