A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|20 VideosSTOICHIOMETRY
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|5 VideosSOLID STATE
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 VideosTHERMODYNAMICS
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|2 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-STOICHIOMETRY-Level 3 - Assertion - Reason Type Questions
- STATEMENTS-1 : Specific gravity is dimensionless. STATEMENTS-2 : Spe...
Text Solution
|
- STATEMENT-1: Molarity of pure water is 55.55 M at 298K. STATEMENT-2 ...
Text Solution
|
- STATEMENT-1: Gram molecular mass of O(2) is 32. STATEMENT-2: Relativ...
Text Solution
|
- STATEMENT-1: The oxidation state of S in H(2)S(2)O(8) is 6. STATEMEN...
Text Solution
|
- STATEMENT-1: 0.1 MH(3)PO(3)(aq) solution has normality equal to 0.3 N...
Text Solution
|
- STATEMENT-1 : MnO(2) can act as an oxidizing agent as well as reducing...
Text Solution
|
- STATEMENT-1 : Equivalent volume of H(2) is 11.2 L at 1 atm and 273 K. ...
Text Solution
|
- STATEMENT-1 : For the reaction in titration Na(2)CO(3)+HCL to NaCl+NaH...
Text Solution
|
- STATEMENT-1 : [Fe(CN)(6)]^(4) to Fe^(3+)+CO(2)+NO(3)^(-), the equivale...
Text Solution
|
- STATEMENT-1 : In the balanced redox reaction, x As(2)S(3)+y NO(3)^(-...
Text Solution
|
- STATEMENT-1 : In the given reaction, NaOH+H(3)PO(4)rarr NaH(2)PO(4)+H(...
Text Solution
|
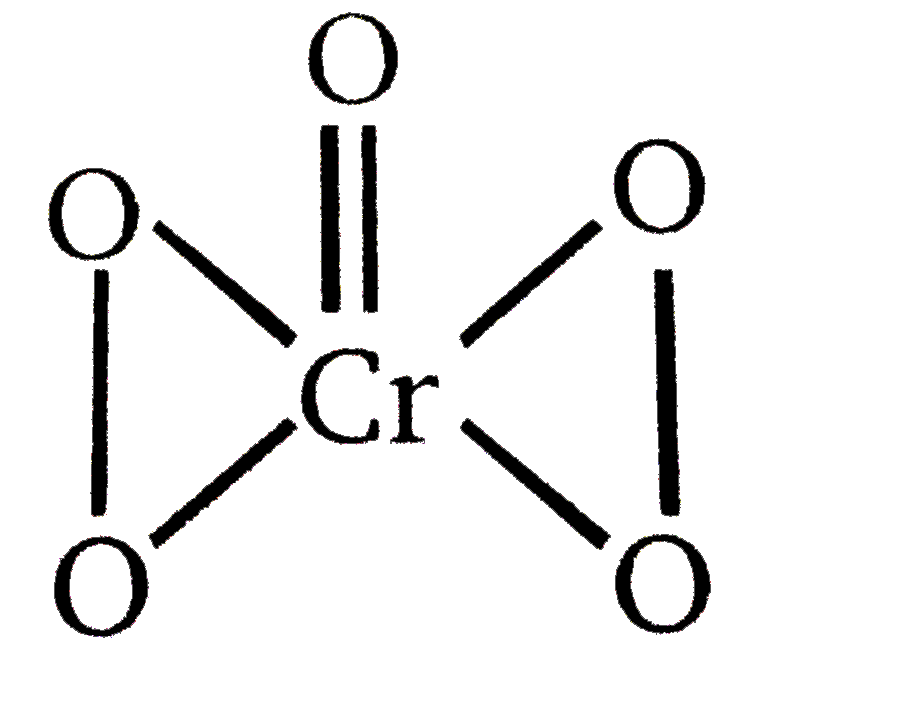
- STATEMENT-1: In CrO(5) oxidation number of Cr is +6. STATEMENT-2 : ...
Text Solution
|
- STATEMENT-1 : I(2)rarrIO(3)^(-)+I^(-), is example of a disproportionat...
Text Solution
|
- Assertion: Fluorine exists only in -1 oxidation state. Reason: Fluor...
Text Solution
|
- STATEMENT-1 : H(2)SO(4) can not act as reducing agent. STATEMENT-2 :...
Text Solution
|