A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- If radius of second stationary orbit (in Bohr's atom) is R then radius...
Text Solution
|
- Which state of the triply ionized Beryllium (Be^(3+)) has the same orb...
Text Solution
|
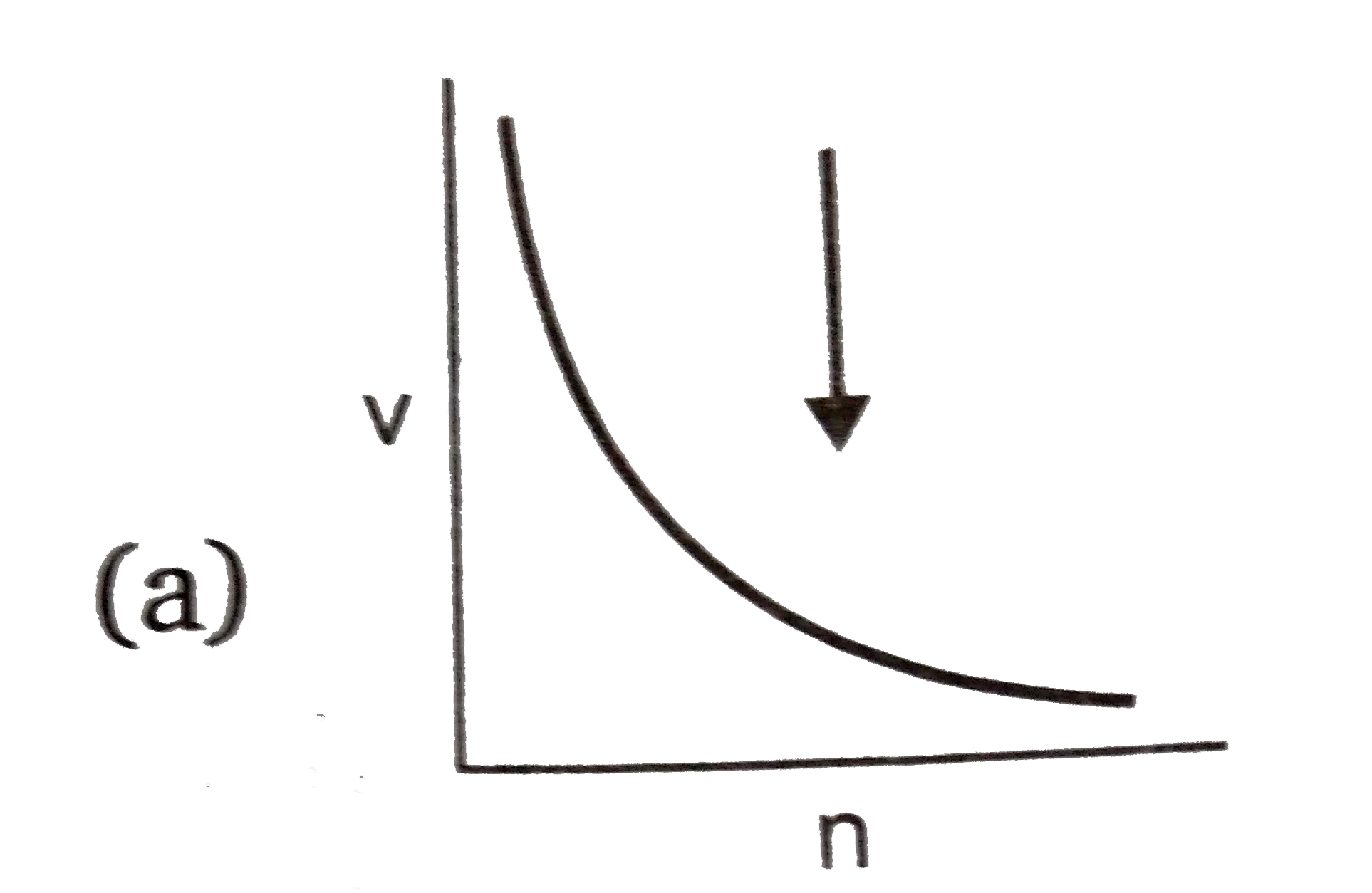
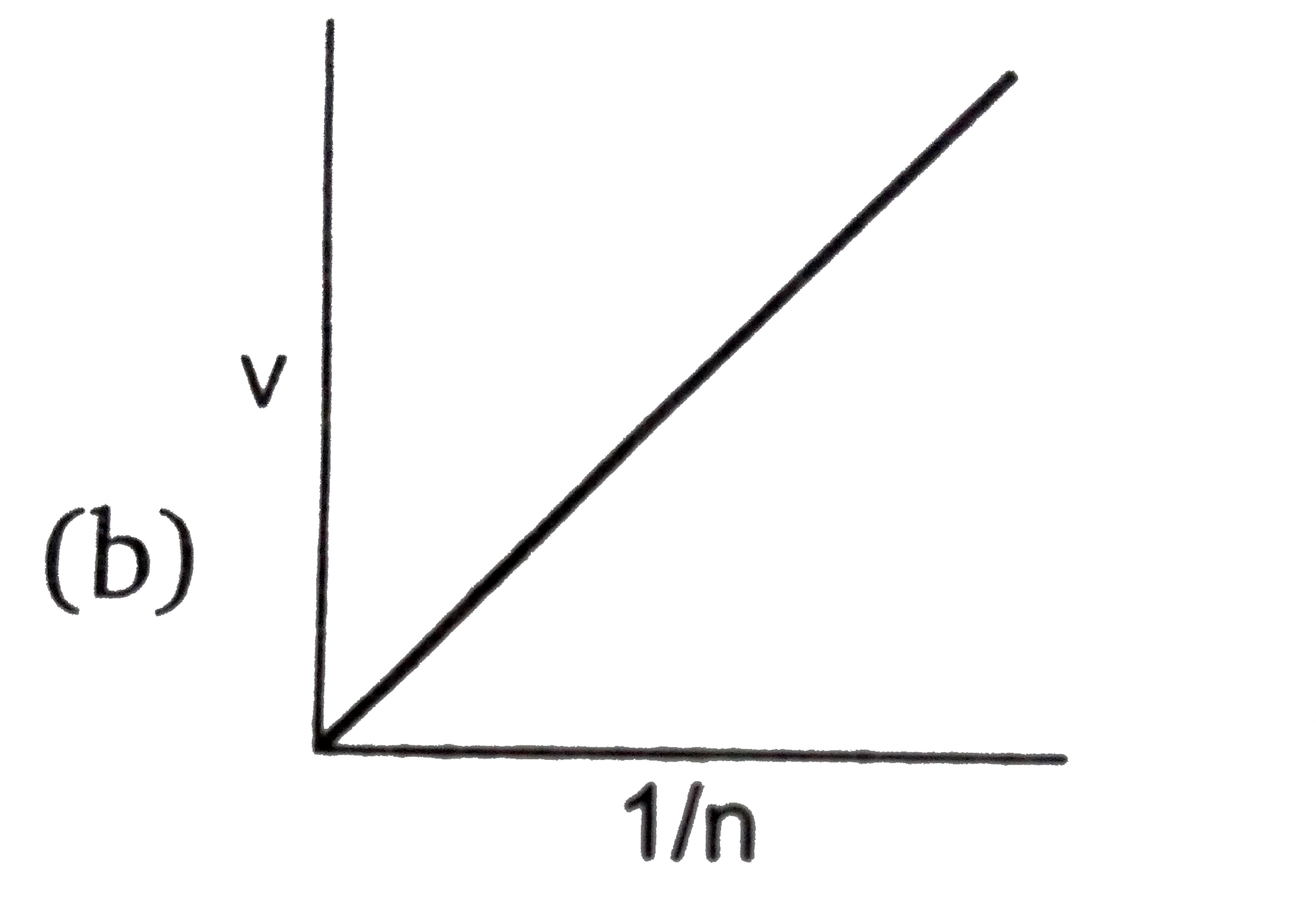
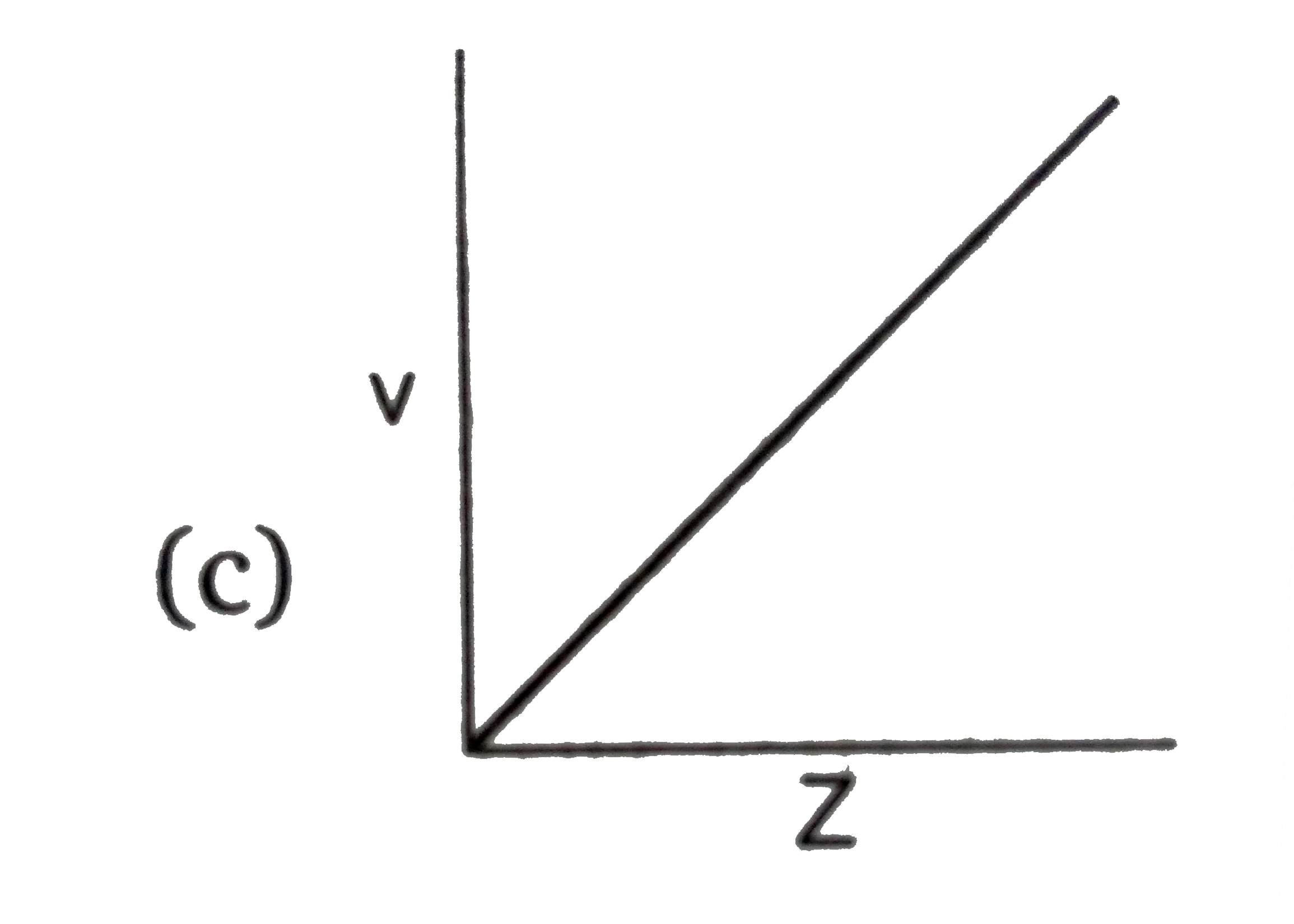
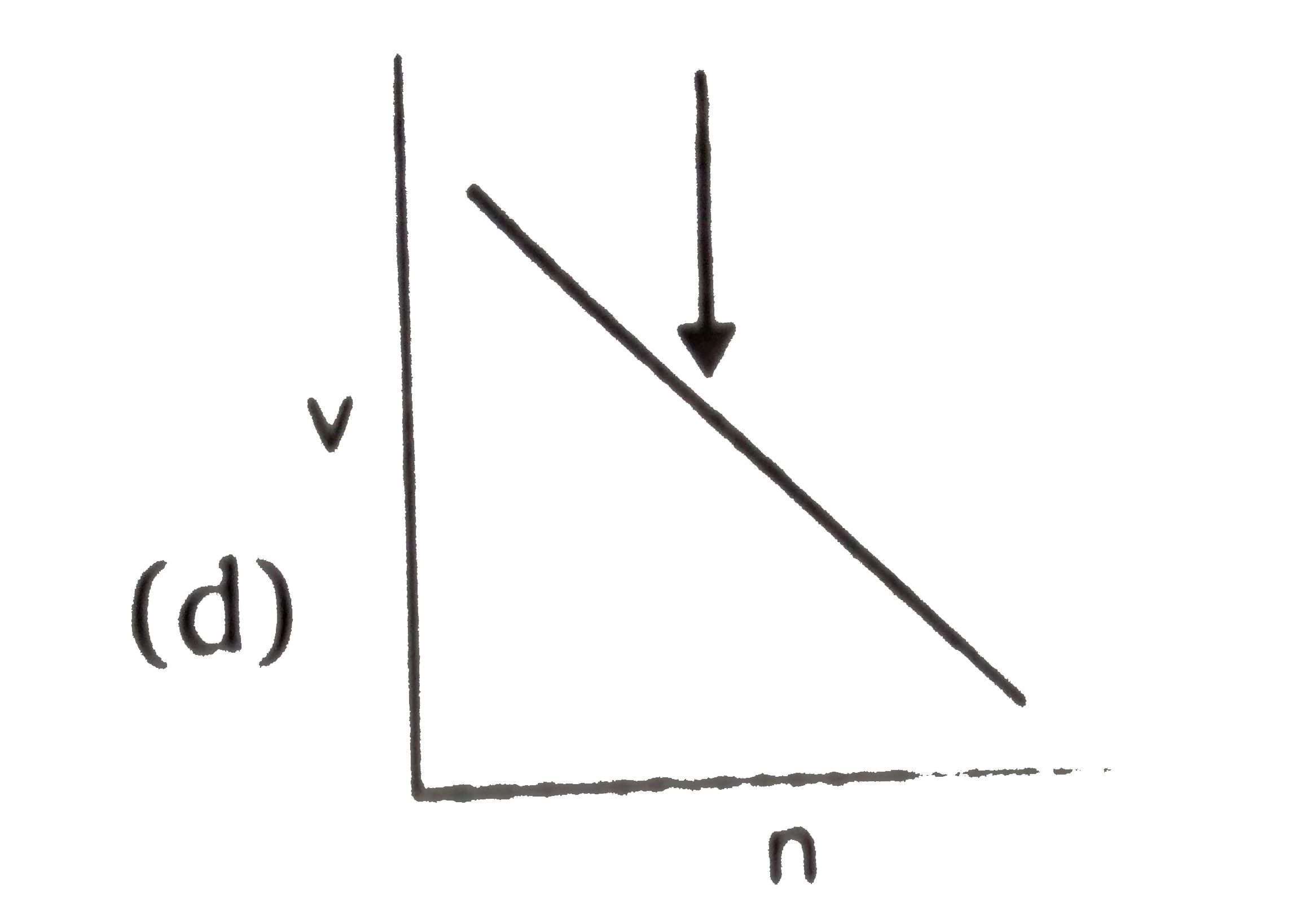
- Select the incorrect graph for velocity of e^(-) in an orbit vs. Z, 1/...
Text Solution
|
- What is the frequency of revolution of electron present in 2nd Bohr's ...
Text Solution
|
- According to Bohr's atomic theory, which of the following is correct ?
Text Solution
|
- Number of waves produced by an electron in one complete revolution in ...
Text Solution
|
- Which of the following statement does not form part of Bohr's model o...
Text Solution
|
- What is the separation energy (in eV ) for Be^(3+) in the first excite...
Text Solution
|
- If in Bohr's model, for unielectronic atom, time period of revolution ...
Text Solution
|
- Which of the following is discreted in Bohr's theory?
Text Solution
|
- What is the ratio of time periods (T(1)//T(2)) in second orbit of hydr...
Text Solution
|
- The mass of an electron is m, charge is e and it is accelerated form r...
Text Solution
|
- If the ionization energy of He^(+) is 19.6xx10^(-18) J per atom then t...
Text Solution
|
- The energy of the second Bohr orbit in the hydrogen atom is -3.41eV. T...
Text Solution
|
- The energy of an electron moving in n^(th) Bohr's orbit of an element ...
Text Solution
|
- If epsilon(0) be the permittivity of vacuum and r be the radius of orb...
Text Solution
|
- Which of the following statement(s) is//are consistent with the Bohr t...
Text Solution
|
- The ionization potential for the electron in the ground state of the h...
Text Solution
|
- What is the energy content per photon (J) for light of frequency 4.2xx...
Text Solution
|
- Wavelength for high energy EMR transition in H-atom is 91 nm. What ene...
Text Solution
|