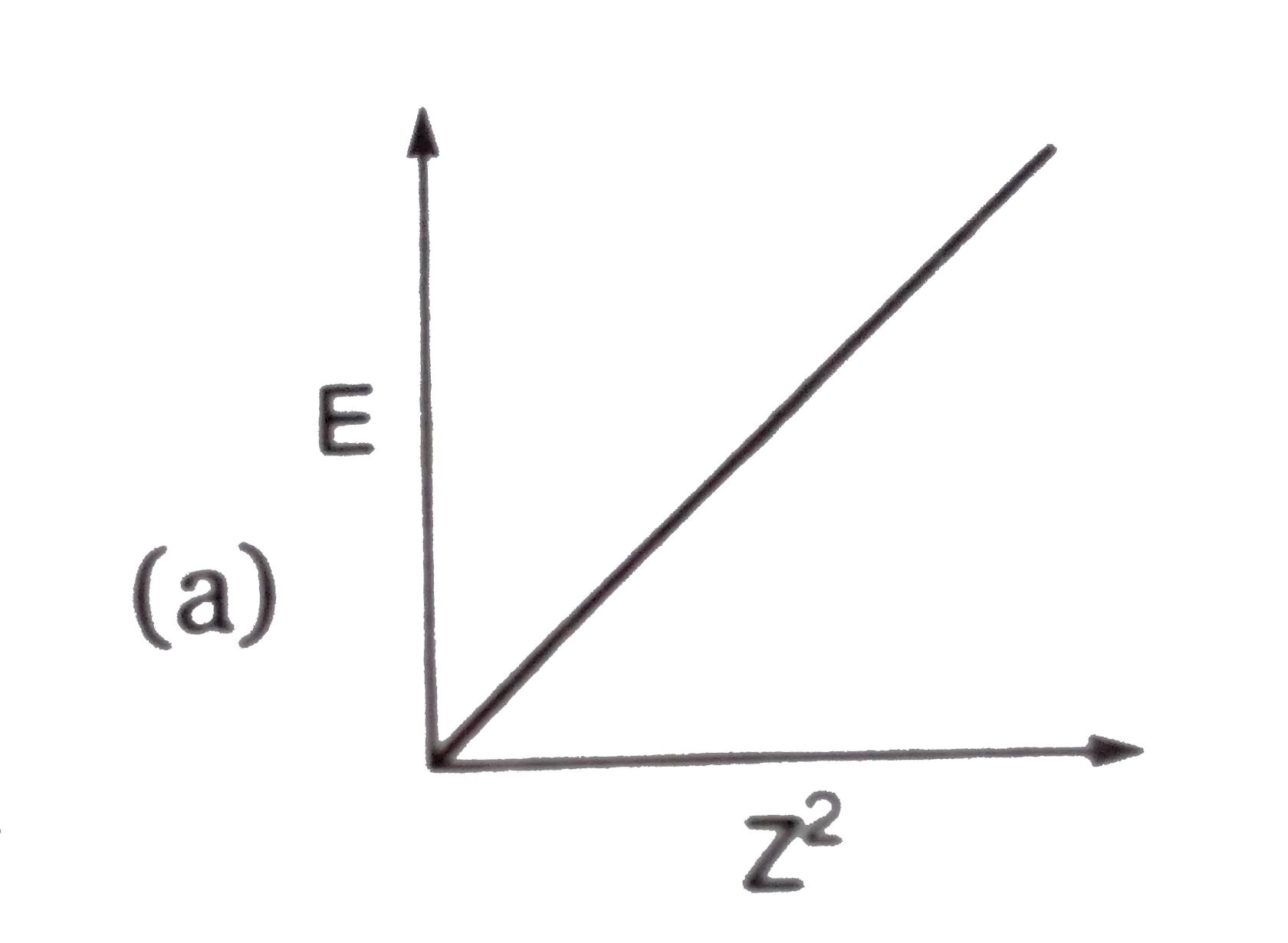
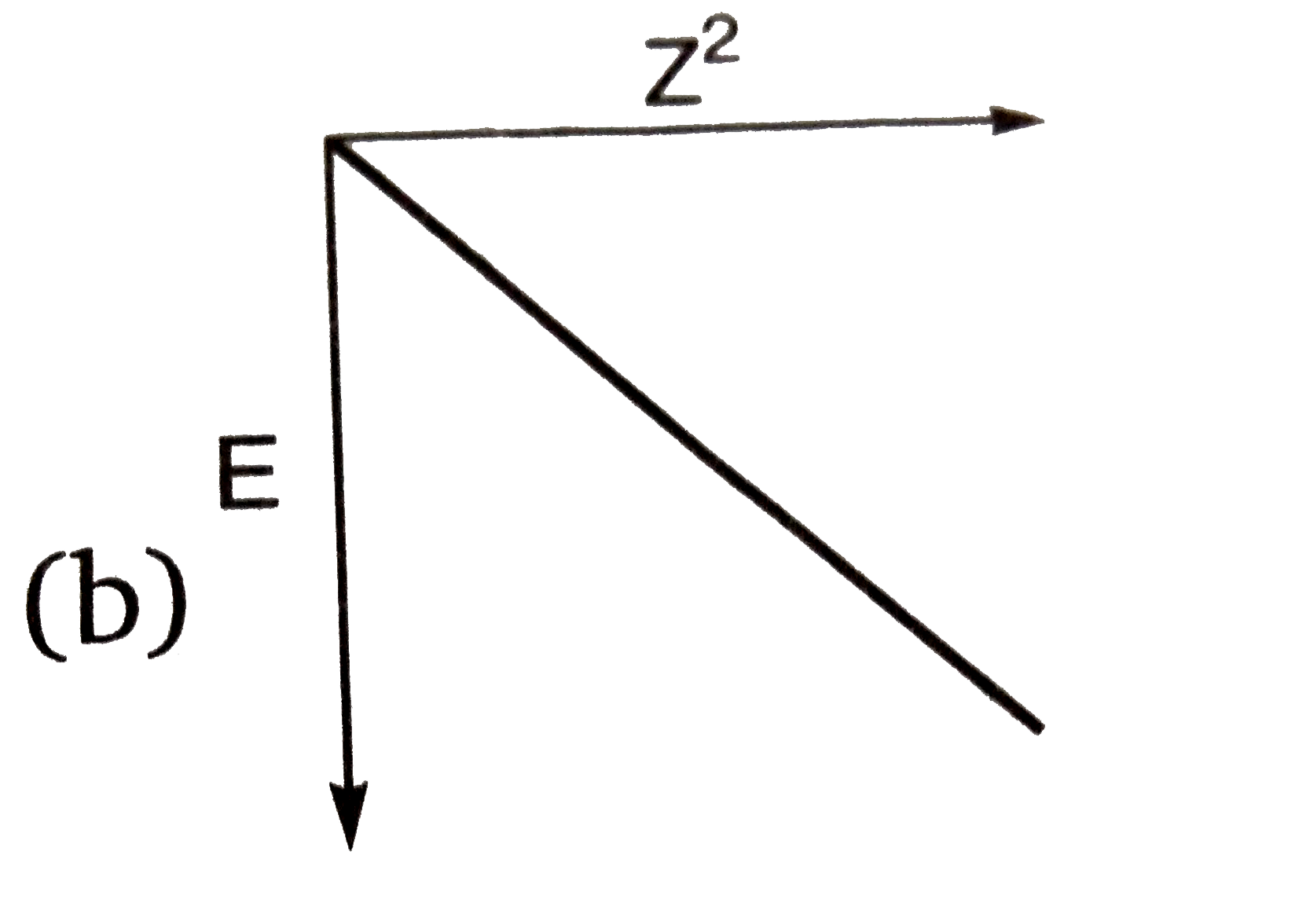
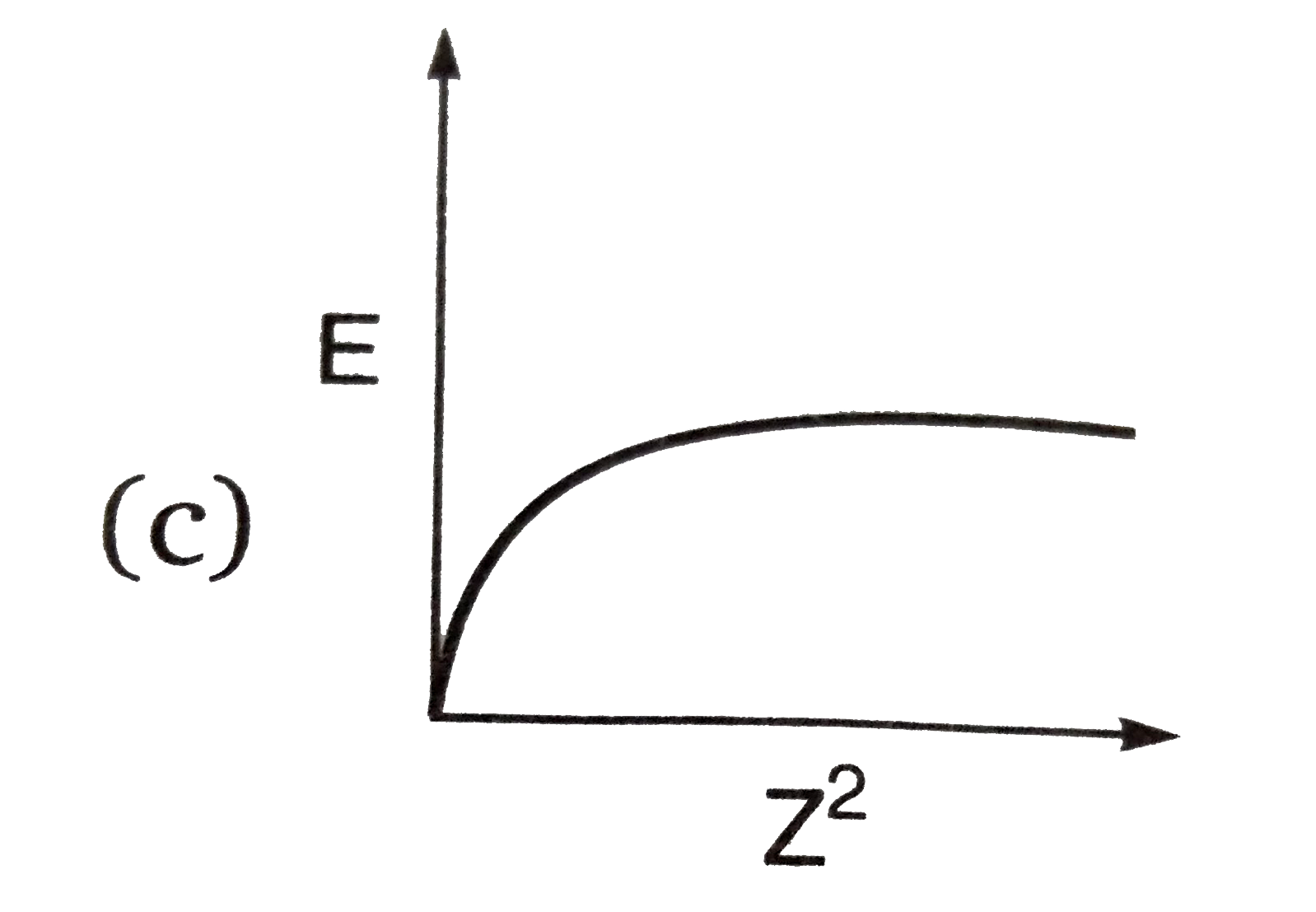
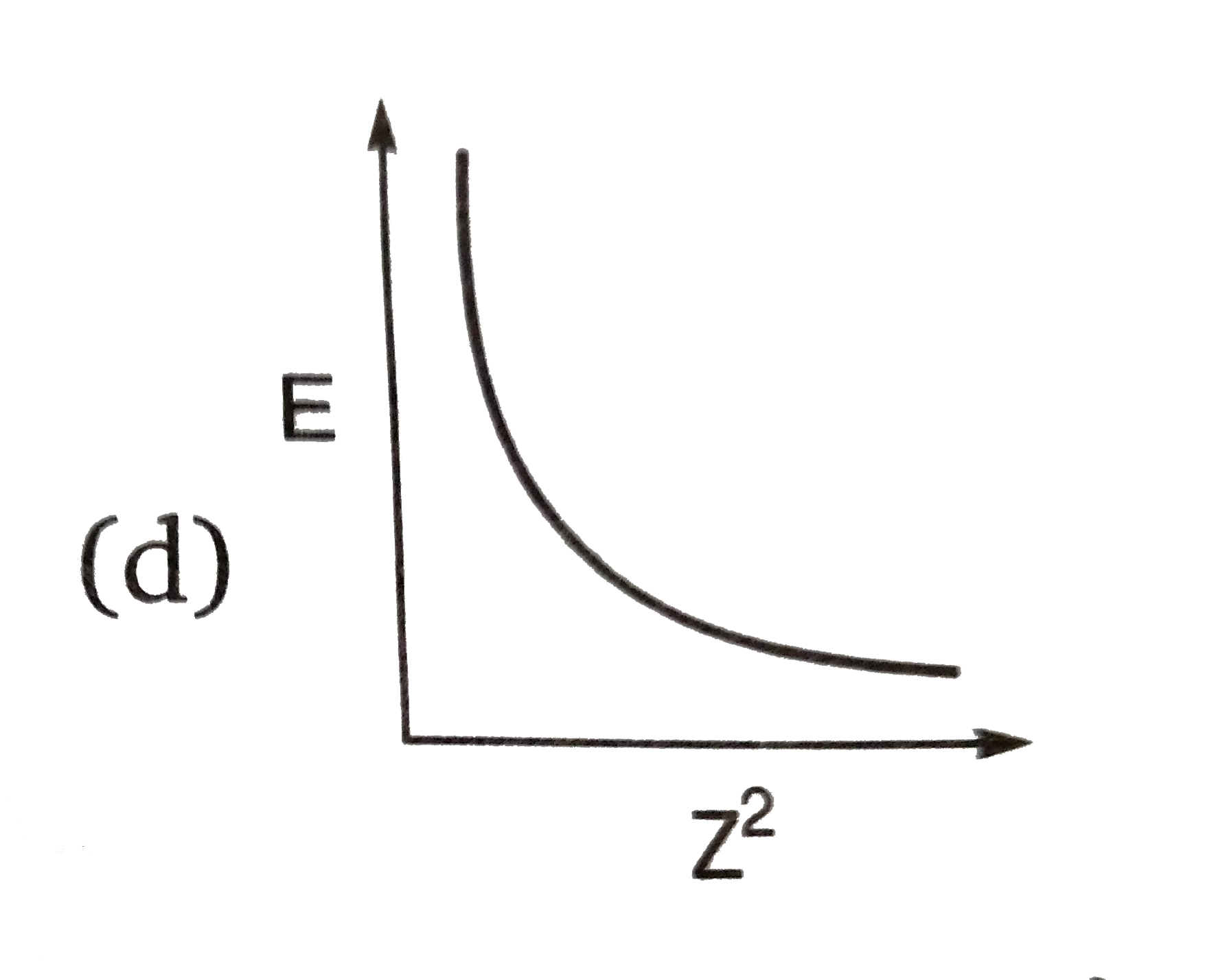
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- If the ionization energy of He^(+) is 19.6xx10^(-18) J per atom then t...
Text Solution
|
- The energy of the second Bohr orbit in the hydrogen atom is -3.41eV. T...
Text Solution
|
- The energy of an electron moving in n^(th) Bohr's orbit of an element ...
Text Solution
|
- If epsilon(0) be the permittivity of vacuum and r be the radius of orb...
Text Solution
|
- Which of the following statement(s) is//are consistent with the Bohr t...
Text Solution
|
- The ionization potential for the electron in the ground state of the h...
Text Solution
|
- What is the energy content per photon (J) for light of frequency 4.2xx...
Text Solution
|
- Wavelength for high energy EMR transition in H-atom is 91 nm. What ene...
Text Solution
|
- Which graph shows how the energy E of a photon of light is related to ...
Text Solution
|
- Assume that 10^(-17)J of light energy is needed by the interior of the...
Text Solution
|
- Line spectra is characteristic of :
Text Solution
|
- The spectrum produced from an element is :
Text Solution
|
- Electronic transition in He^(+) ion takes from n(2) " to " n(1) shell ...
Text Solution
|
- Which of the following expressions represents the spectrum of Balmer s...
Text Solution
|
- Multiple or fine structure of spectral lines is due to :
Text Solution
|
- Whith increasing principal quantum number, the energy difference betwe...
Text Solution
|
- Find the value of wave number (overset-v) in terms of Rydberg's consta...
Text Solution
|
- What is the wavelength in nm of the spectral line associated with a tr...
Text Solution
|
- What is the energy (kJ/mol) associated with the de-excitation of an el...
Text Solution
|
- What is the shortest wavelength line in the Paschen series of Li^(2+) ...
Text Solution
|