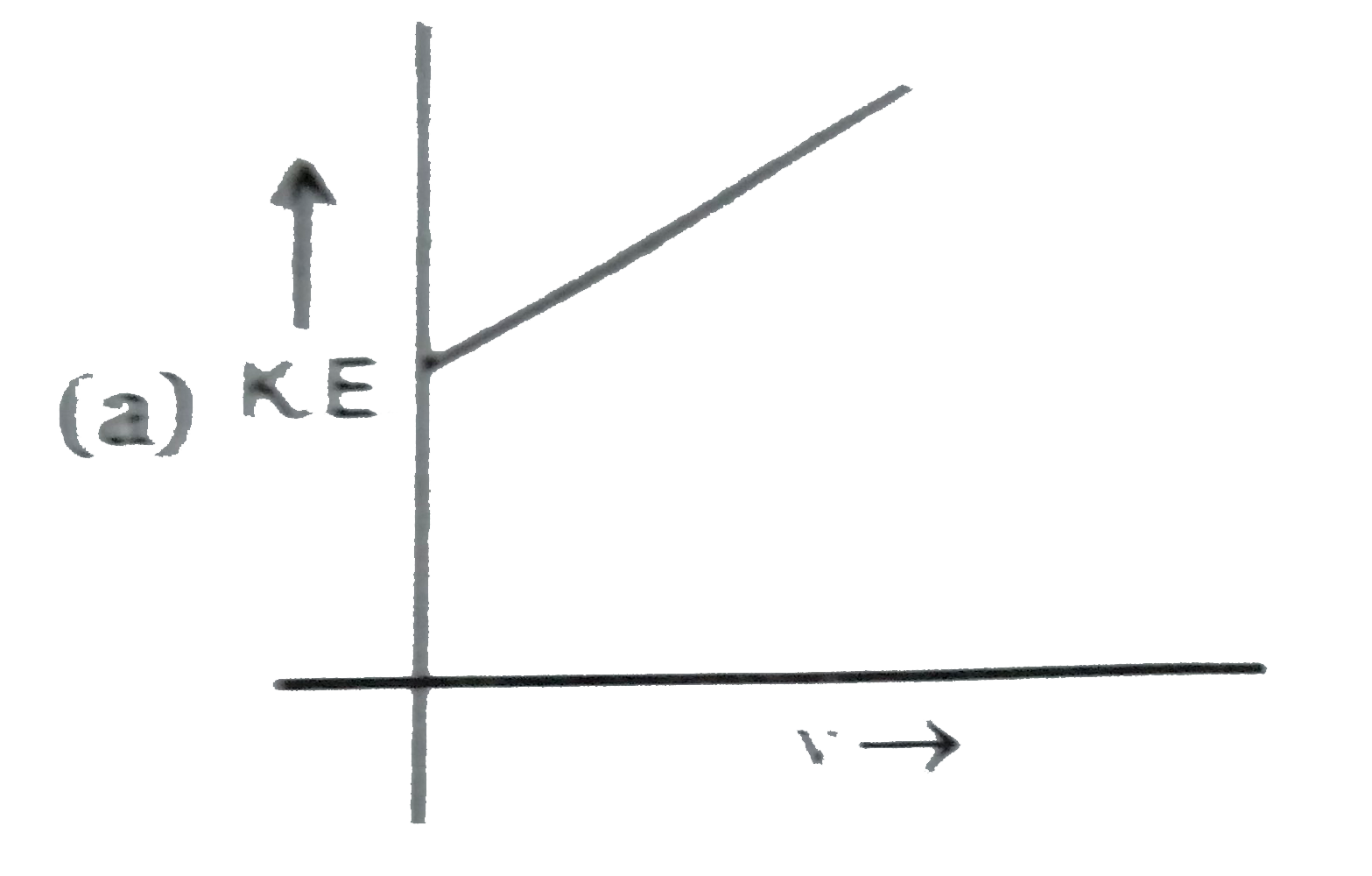
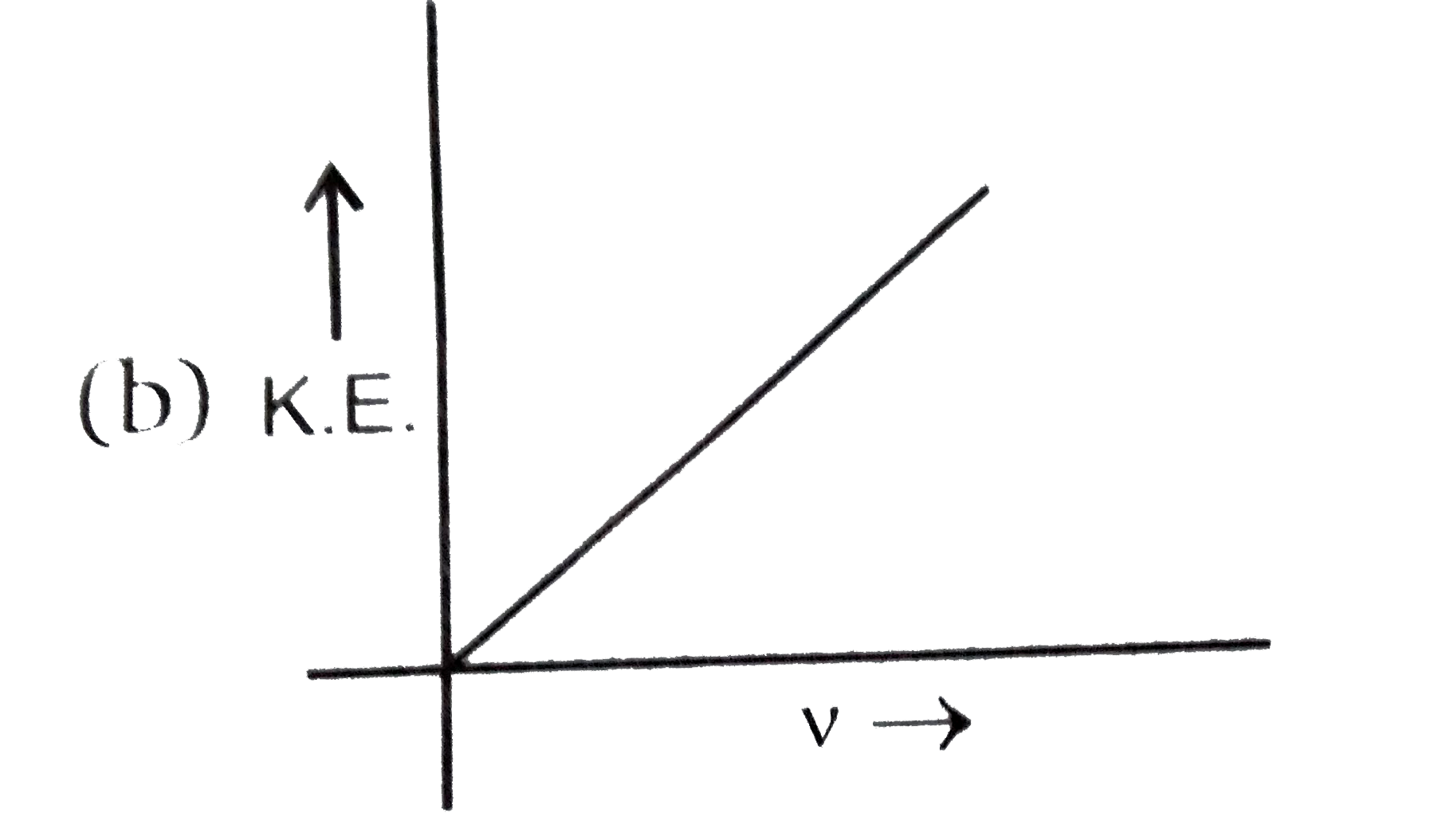
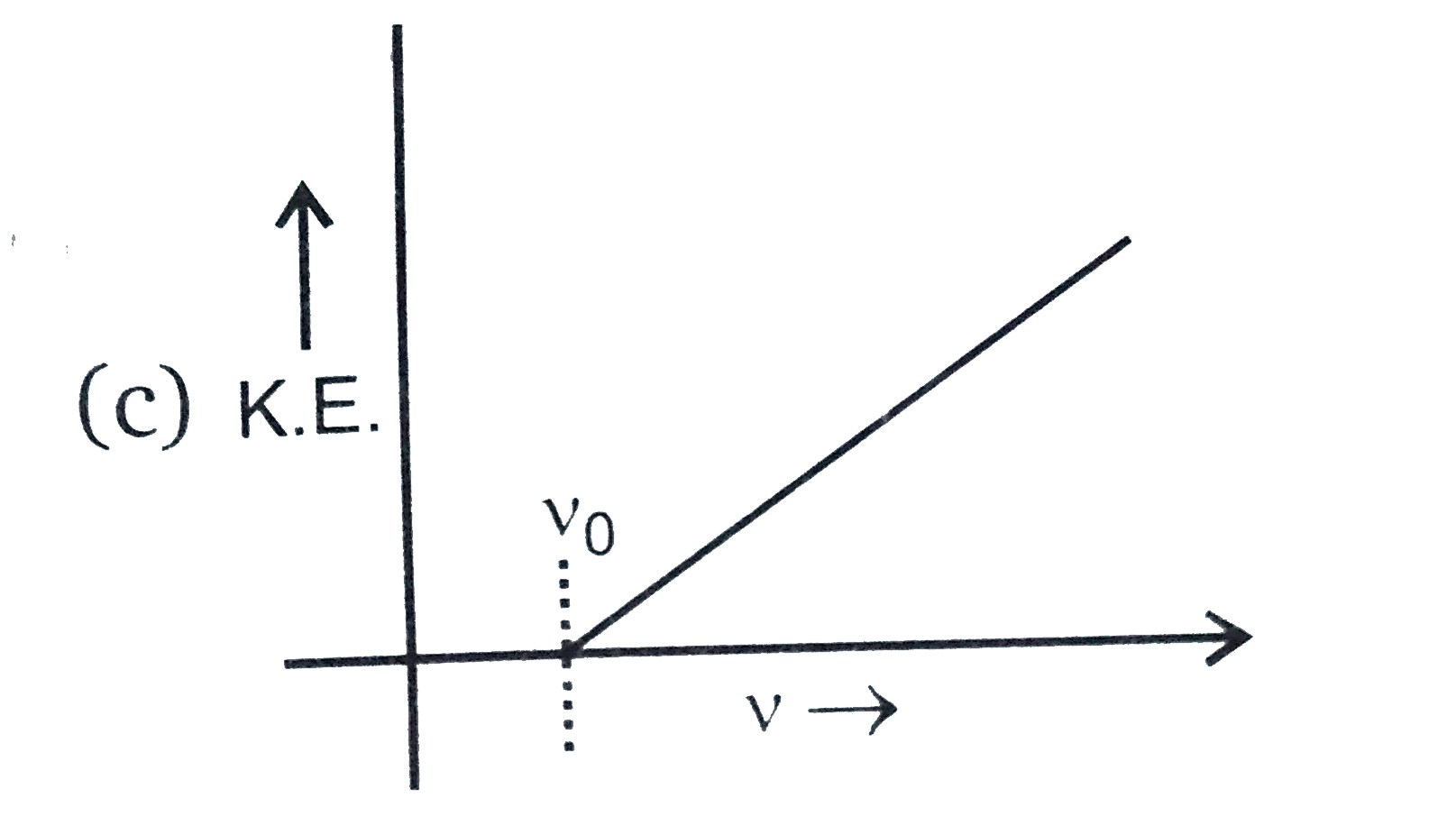
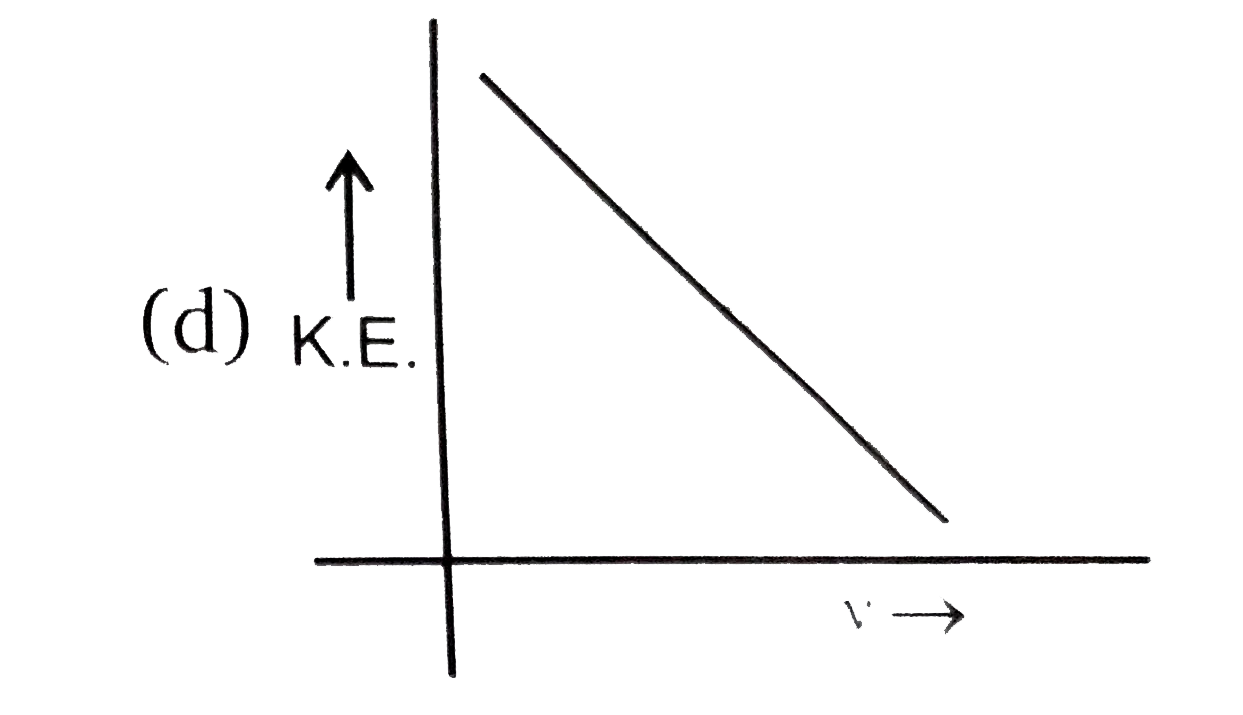
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- In photoelectric effect, the number of photoelectrons emitted is propo...
Text Solution
|
- Slope of V(0) vs v curve is (where V(0)= Stopping potential, v=subject...
Text Solution
|
- According to Einstein's photoelectric equation, the graph between kine...
Text Solution
|
- The photoelectric emission from a surface starts only when the light i...
Text Solution
|
- If lamdao and lamda be the threshold wavelength and wavelength of inci...
Text Solution
|
- A light source of wavelength lambda illuminates a metal and ejects pho...
Text Solution
|
- Electronmagnetic radiations having lambda=310Åare subjected to a metal...
Text Solution
|
- The ratio of slopes of K(max) vs. V and V(0) vs. v curves in the photo...
Text Solution
|
- Radiation corresponding to the transition n=4 to n=2 in hydrogen atoms...
Text Solution
|
- Select the incorrect statement.
Text Solution
|
- Which is the de-Broglie equation?
Text Solution
|
- Which of the following has the largest de Broglie wavelength (all have...
Text Solution
|
- The de-Broglie wavelength associated with a particle of mass 10^-6 kg ...
Text Solution
|
- For two particles A and B, curves are plotted sqrtV against de-Broglie...
Text Solution
|
- Which of following graphs correctly represents the variation of partic...
Text Solution
|
- An excited state of H atom emits a photon of wavelength lamda and retu...
Text Solution
|
- A dye absorbs a photon of wavelength lambda and re- emits the same ene...
Text Solution
|
- Be^(3+) and a proton are accelerated by the same potential, their de-...
Text Solution
|
- de Broglie wavelength of an electron after being accelerated by a pote...
Text Solution
|
- An electron travels with a velocity of x ms^(-1). For a proton to have...
Text Solution
|