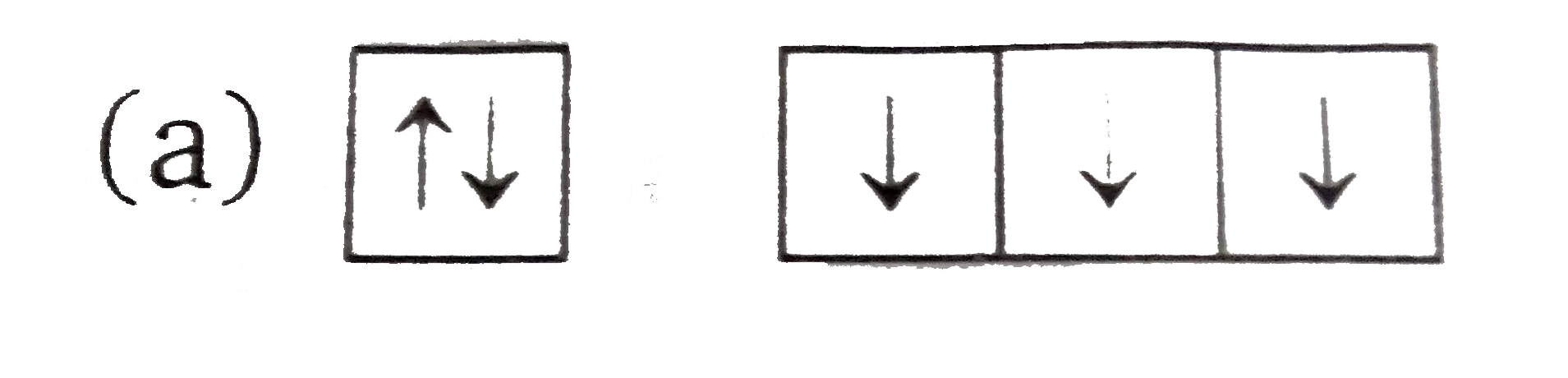
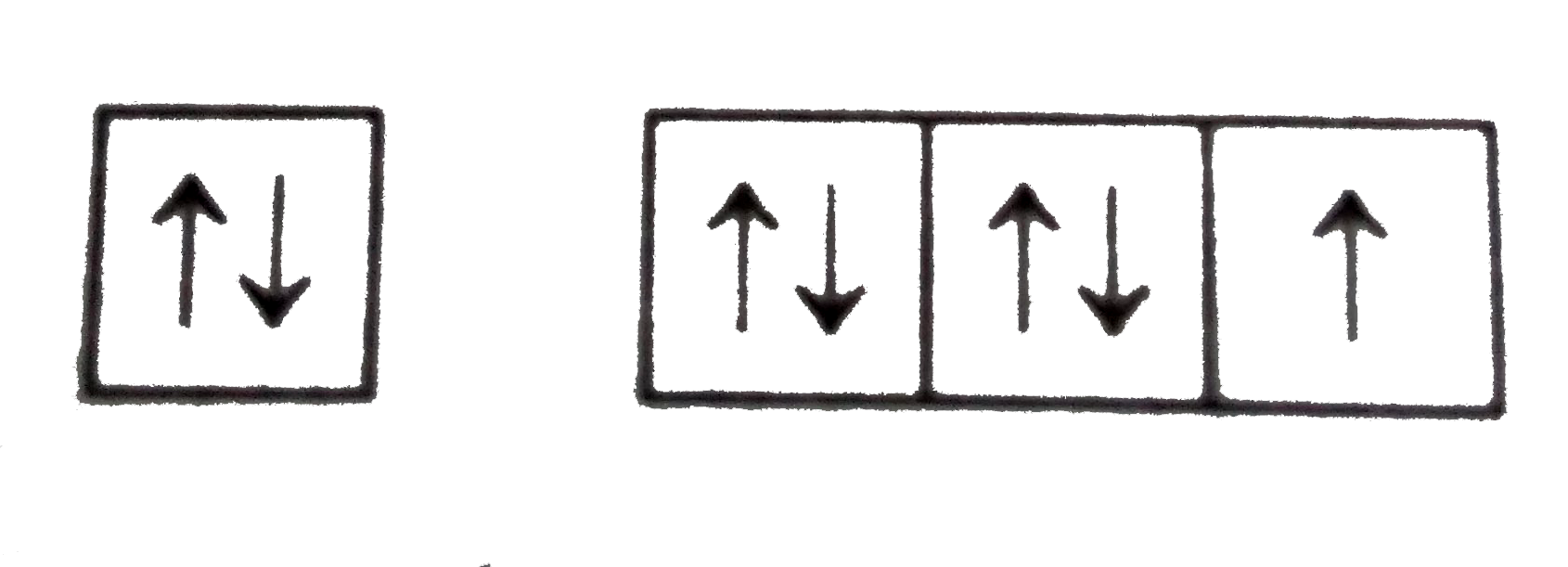
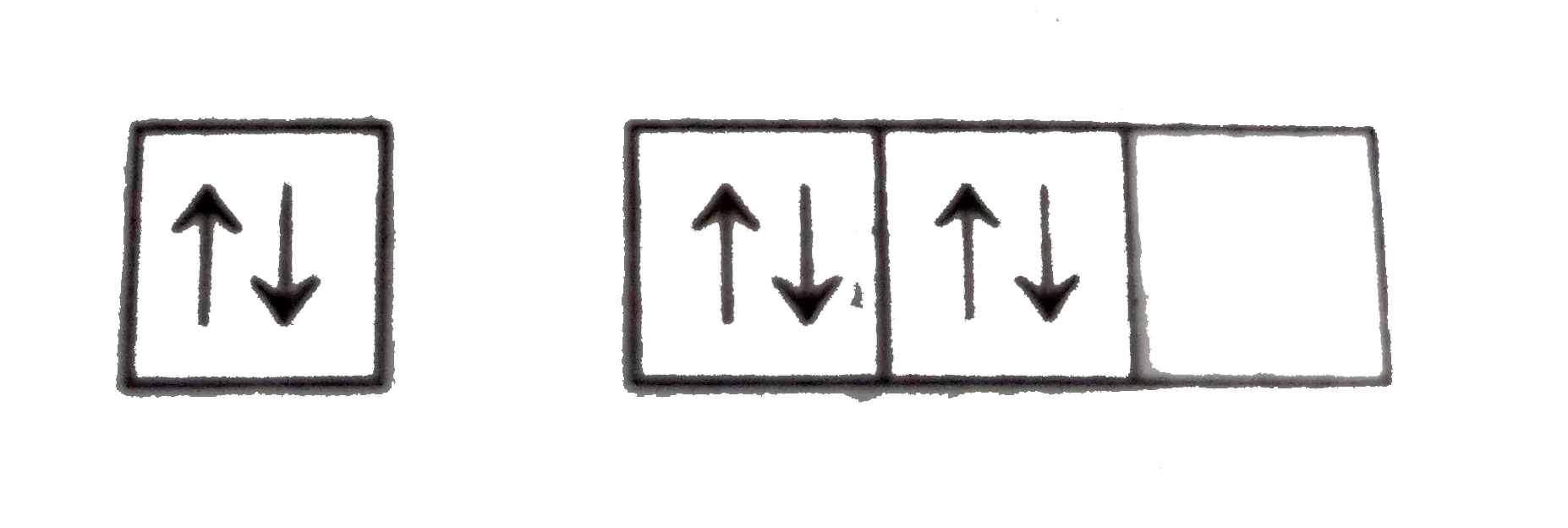
A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- The orbital angular momentum of 3p electron is :
Text Solution
|
- The atomic orbitals are progerssively filled in order of increasing en...
Text Solution
|
- The orbital diagram in which both Pauli's exclusion principle and Hund...
Text Solution
|
- Which of the following elements is represents by the electronic config...
Text Solution
|
- The ratio of magnetic of Fe (III) and Co (II) is :
Text Solution
|
- If the electronic structure of oxygen atom is written as 1s^(2) , 2s^(...
Text Solution
|
- A compound of vanadium has a magneitc moment (mu) of 1.73 BM. If the v...
Text Solution
|
- d^(6) configuration will result in total spin of :
Text Solution
|
- The probability of finding electron in d(xy) orbital is :
Text Solution
|
- Select correct statement :
Text Solution
|
- Read the following statements and choose the correct option. (I) ...
Text Solution
|
- The quantum number of four electrons (el to e4) are given below :- ...
Text Solution
|
- The energy of an electron of 2p(x) orbital is :
Text Solution
|
- In group 15 elements, the number of unpaired electrons in valence shel...
Text Solution
|
- The orientation of an orbital is governed by the quantum number k...
Text Solution
|
- What is the maximum number of electrons in a subshell that can have th...
Text Solution
|
- which of the following statements about an electron with m1=+2 is inco...
Text Solution
|
- which of the following set of quantum numbers is impossible for an ele...
Text Solution
|
- In a 3d subshell, all the five oprbitals are degenerate. What does it ...
Text Solution
|
- which of the following subshell can accommodate as many as 10 electron...
Text Solution
|