A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- Which of the following has the maximum number of unpaired electrons?
Text Solution
|
- Which of the following orbitals has two spherical nodes?
Text Solution
|
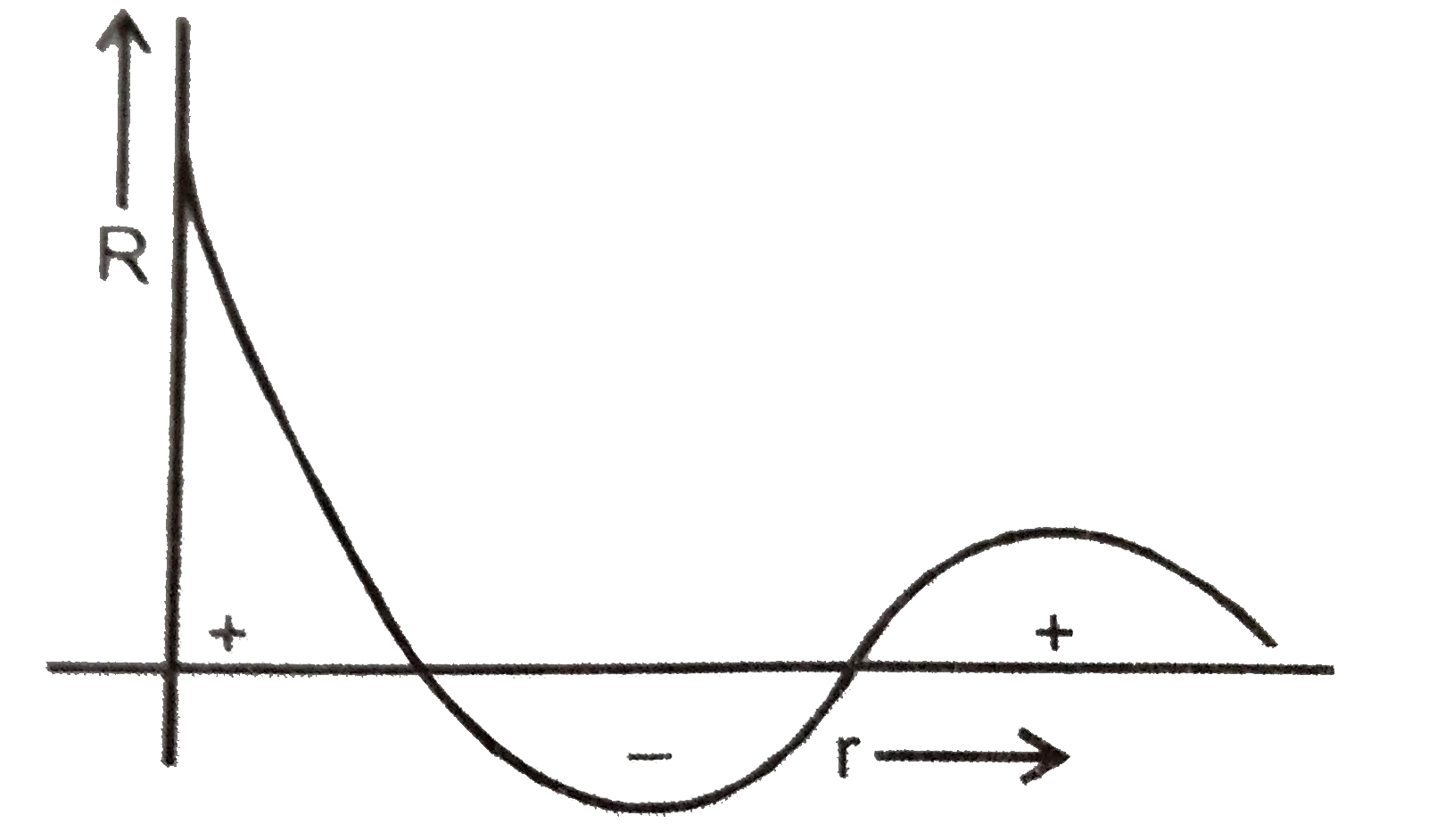
- Wave function of an orbital is plotted against the distance from nucle...
Text Solution
|
- The Schrodinger wave equation for hydrogen atom is Psi(2s) = (1)/(4sqr...
Text Solution
|
- The Schrodinger wave equation for hydrogen atom is Psi("radial")=(1)...
Text Solution
|
- Potential energy of electrom present in He^(+) is :
Text Solution
|
- A single electron in an ion has ionization energy equal to 217.6eV. Wh...
Text Solution
|
- For a hypothetical hydrogen like atom, the potential energy of the sys...
Text Solution
|
- A beam of specific kind of particles of velocity 2.1 xx 10^7 m//s is s...
Text Solution
|
- What is the angular velocity (omega) of an electron occupying second o...
Text Solution
|
- The ratio of the radius difference between 4^(th) and 3^(rd) orbit of ...
Text Solution
|
- The velocity of an e in excited state of H-atom is 1.093 xx 10^(6)m//s...
Text Solution
|
- The angular momentum of an electron in a Bohr's orbit of He^(+) is 3.1...
Text Solution
|
- If radiation corresponding to second line of "Balmer series" of Li^(+2...
Text Solution
|
- When an electron makes a transition from (n+1) state of nth state, the...
Text Solution
|
- In a collection of H-atoms, all the electrons jump from n=5 to ground ...
Text Solution
|
- An electron is allowed to move freely in a closed cubic box of length ...
Text Solution
|
- An element undergoes a reaction as shown sx+2e^(-)tox^(-2) Energy r...
Text Solution
|
- Ground state energy of H-atom is (-E(1)),t he velocity of photoelectro...
Text Solution
|
- At which temperature will the translational kinetic energy of H-atom e...
Text Solution
|