A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- Select incorrect statement (s) :
Text Solution
|
- Select the correct statement (s) :
Text Solution
|
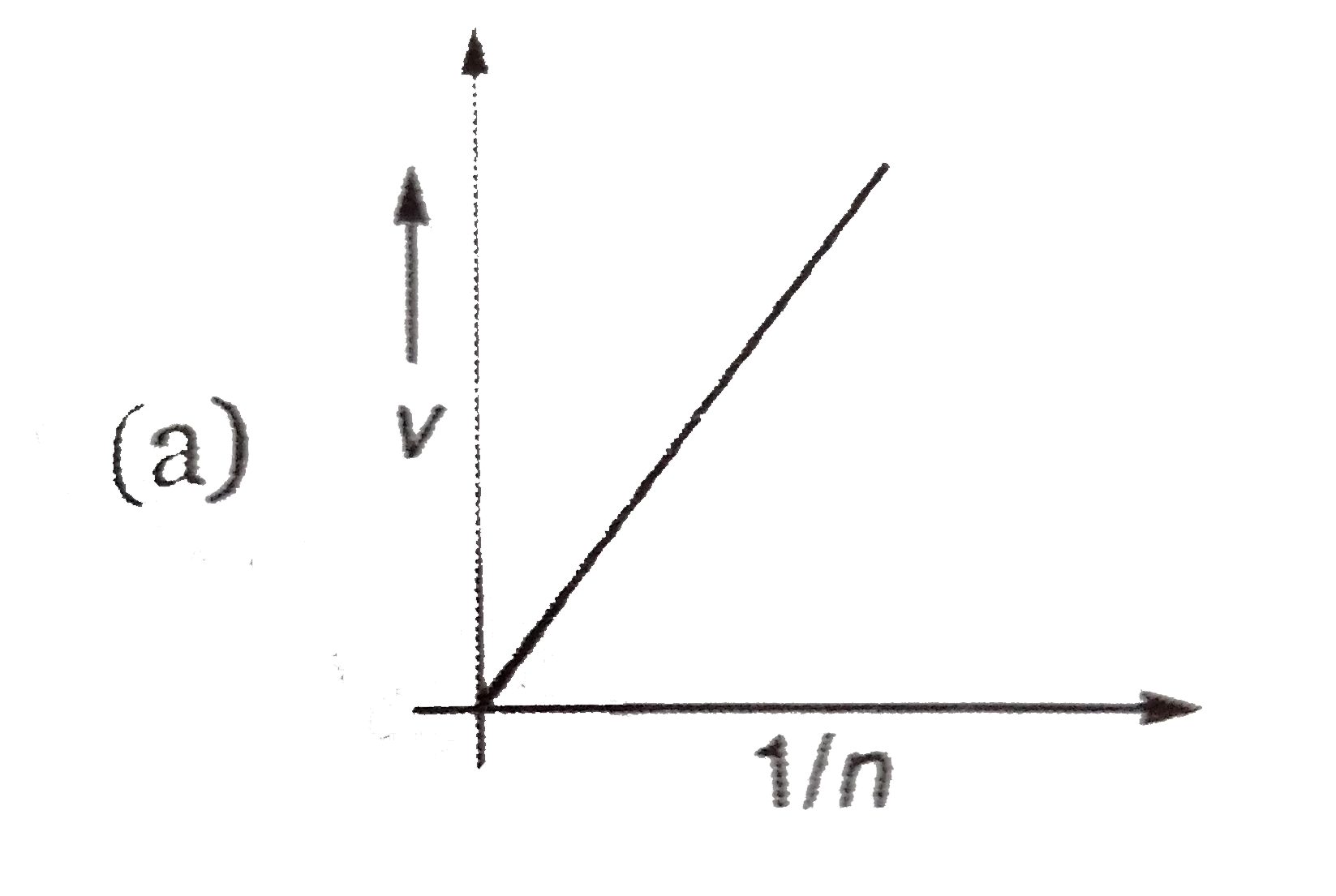
- Select the correct curve (s) : If = V Velocituy of electron in Bohr'...
Text Solution
|
- Select the correct set (s) of quantum numbers
Text Solution
|
- Which is /are correct statement ?
Text Solution
|
- In a sample of H-atoms electrons are de-exicited from 4^(th) excited s...
Text Solution
|
- Column-I and Column-II contains fore entries each. Entries of Column-I...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"Thomson model of atom",(P),"Electrons...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),(K.E.)/(P.E.),(P),2),((B),P.E+2K.E.,(Q...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"Lyman series",(P),"Visible region"),(...
Text Solution
|
- In case of hydrogen spectrum wave number is given by barv=R(H)[(1)/(...
Text Solution
|
- {:("ColumnI","ColumnII"),((A)2nd,(P)1),((B)3rd,(Q)2),((C)4th,(R)3),((D...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"The radial node of 5s atomic orbital ...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"The d-orbital which has two angular n...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"Orbital angular momentum of an electr...
Text Solution
|
- {:(,"ColumnI",,"ColumnII"),((A),"Number of orbitials in then" n^(th)"s...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|