A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ATOMIC STUCTURE-Exercise
- STATEMENT-1: Half-filled and fully-filled degenerate orbitals are more...
Text Solution
|
- Statement-I : The ground state configuration of Cr is 3d^(5) 4s^(1). ...
Text Solution
|
- STATEMENT-1: The ground state electronic configuration of introgen is ...
Text Solution
|
- STATEMENT-1: An orbital cannot have more then two electrons and they m...
Text Solution
|
- STATEMENT-1: Orbital having xz plane as nofe may be 3d(xy) STATEMENT...
Text Solution
|
- STATEMENT-1: The kinetic energy of photo-electrons increases with incr...
Text Solution
|
- Assertion : Cu^(2+) ion is a coloured ion . Reason : Every ion with...
Text Solution
|
- Given r(n+1)-r(n-1)=2r(n),where r(n)-r(n-1)=r(n+1), are Bohr radius fo...
Text Solution
|
- The energy of separationof an of an electron is 30.6eV moving in an or...
Text Solution
|
- Calculate the number of waves made by a Bohr electyron in one complete...
Text Solution
|
- A certain day absorbs lights of lamda=400 nm and then fluorescence lig...
Text Solution
|
- Aphoton of energy 4.5 eV strikes on a metal surface of work function 3...
Text Solution
|
- Find out the difference in number of angular nodes and number of radia...
Text Solution
|
- What is the total numbe of radial and angular nodes present in 5f orbi...
Text Solution
|
- Infrared lamps are used in restaurants to keep the food warm. The infr...
Text Solution
|
- When an electron makes transition from (n+1)state to n state the wavel...
Text Solution
|
- For 3s orbital of hydrogen atom, the normalised wave function is Psi...
Text Solution
|
- Find the separation between two electron (inÅ) in vacuum, if electrost...
Text Solution
|
- An alpha - [article moving with velocity (1)/(30) th times of velveloc...
Text Solution
|
- In a sample of excited hydrogen atoms electrons make transition from n...
Text Solution
|
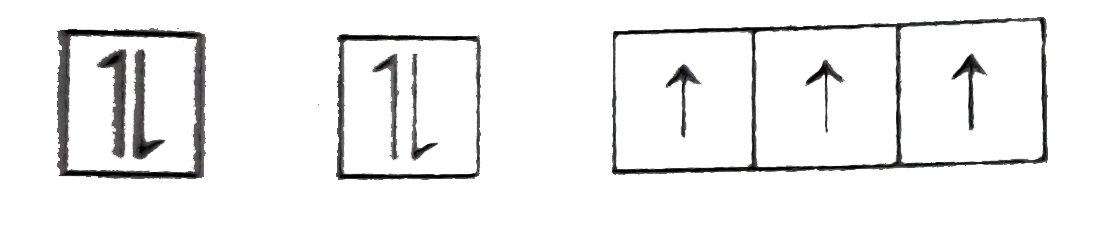 STATEMENT-2: Electronic are filled in orbitals as per aufbau principle, Hund's rule of maximum spin multiplicity and puli's principle.
STATEMENT-2: Electronic are filled in orbitals as per aufbau principle, Hund's rule of maximum spin multiplicity and puli's principle.