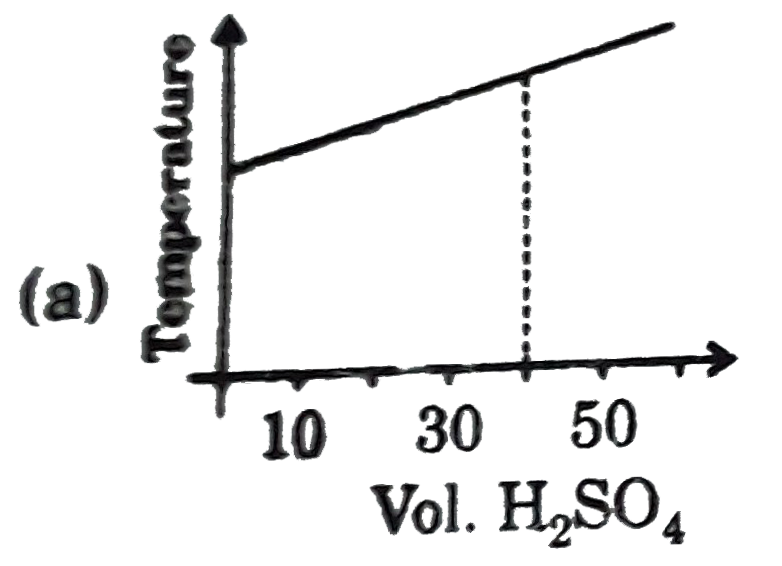
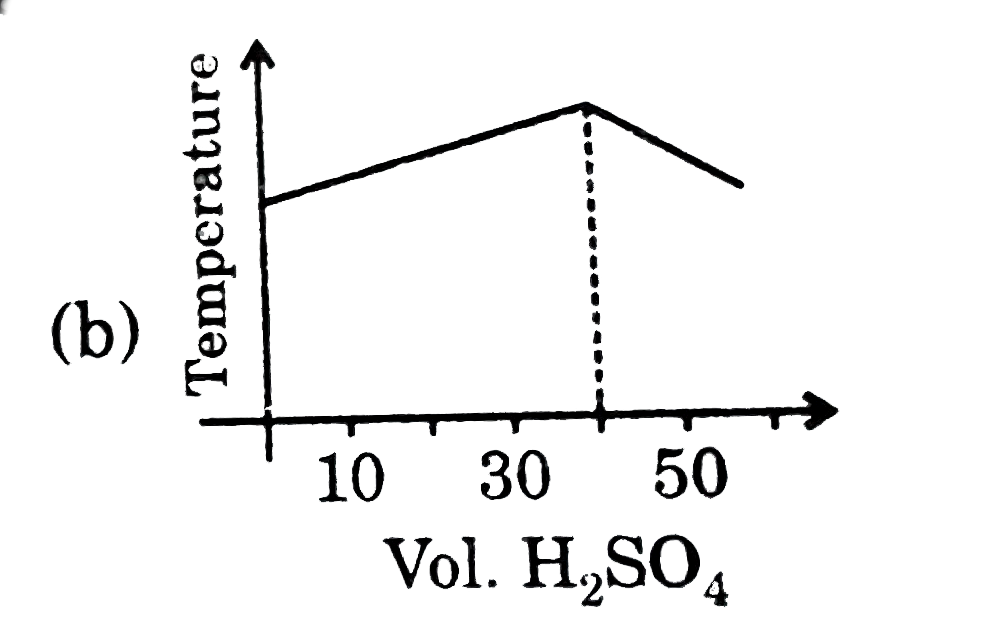
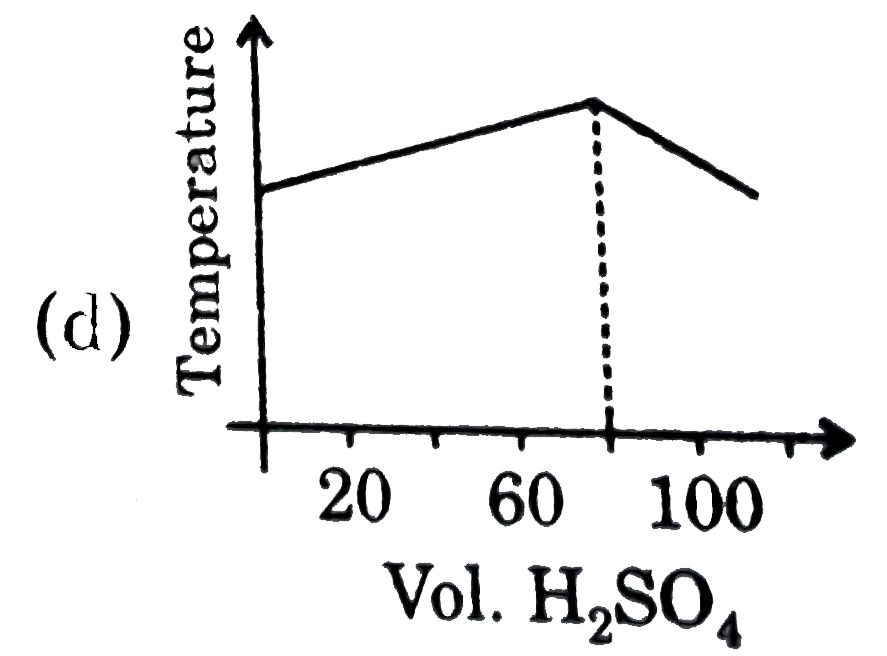
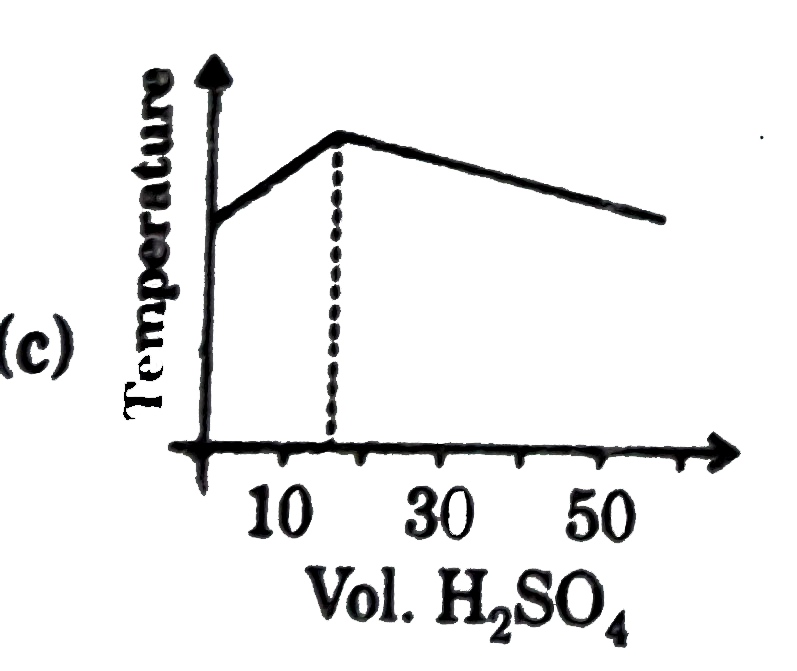A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise B. Gaseous State|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Gaseous State|39 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Subjective Type|23 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Thermodynamics
- Calculate magnitude of work done in calorie for one mole of an ideal g...
Text Solution
|
- An ideal gas in subjected to a three step reversible process as shown....
Text Solution
|
- In an experiment to determine the enthalpy of neutralisation of sodium...
Text Solution
|
- Chemicals A and B each initially at 20^(@)C react exothermically. A gr...
Text Solution
|
- An ideal gas is subjected to various changes which are plotted as foll...
Text Solution
|
- A thermodynamic process is shown in the following figure. In the proce...
Text Solution
|
- Which of the following graph is correct reversible adiabatic process f...
Text Solution
|
- Which process is identical with the cyclic process shown below ? If AB...
Text Solution
|
- An ideal gas is subjected to two different process in which it is heat...
Text Solution
|
- Which is correct for cyclic process as shown in figure ? Choose the ...
Text Solution
|
- For an ideal gas three adiabatic processes are carried out upto same f...
Text Solution
|
- A student is calculating the work during a reversible isothermal proce...
Text Solution
|
- [At constant temperature and pressure] Statement-I : C will part...
Text Solution
|
- If the internal energy of an ideal gas varies as U = 2PV and the gas u...
Text Solution
|
- This curve is produced when a pure substance is heated. Which characte...
Text Solution
|
- What can be concluded about the values of DeltaH and DeltaS from this ...
Text Solution
|
- The process in which an ideal gas undergoes change from X to Y as show...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- A heat engine carries one mole of an ideal monoatomic gas around the c...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|