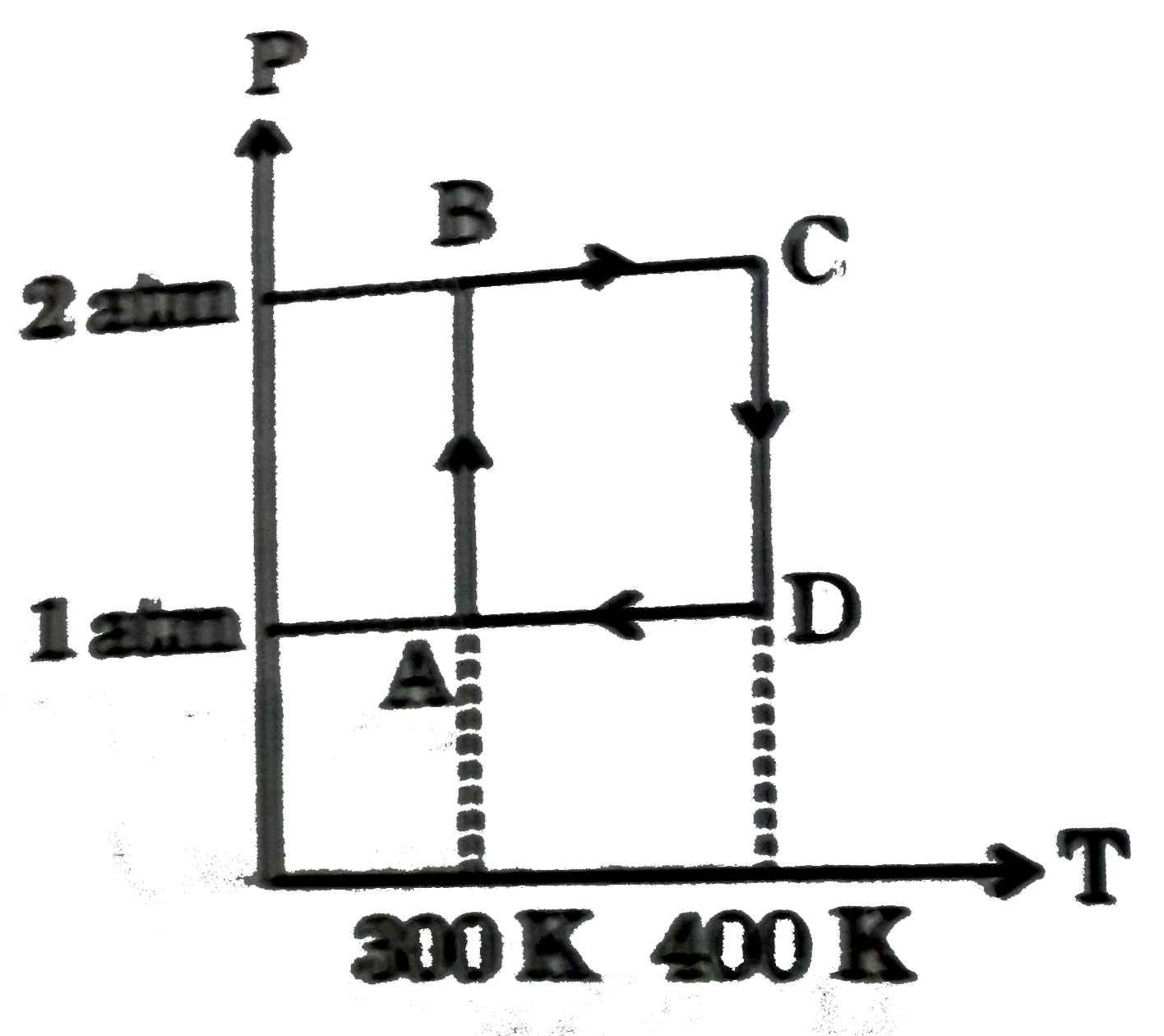
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise B. Gaseous State|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Gaseous State|39 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Subjective Type|23 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Thermodynamics
- An ideal gas is subjected to two different process in which it is heat...
Text Solution
|
- Which is correct for cyclic process as shown in figure ? Choose the ...
Text Solution
|
- For an ideal gas three adiabatic processes are carried out upto same f...
Text Solution
|
- A student is calculating the work during a reversible isothermal proce...
Text Solution
|
- [At constant temperature and pressure] Statement-I : C will part...
Text Solution
|
- If the internal energy of an ideal gas varies as U = 2PV and the gas u...
Text Solution
|
- This curve is produced when a pure substance is heated. Which characte...
Text Solution
|
- What can be concluded about the values of DeltaH and DeltaS from this ...
Text Solution
|
- The process in which an ideal gas undergoes change from X to Y as show...
Text Solution
|
- An ideal gas is taken around the cycle ABCA as shown in P-V diagram. T...
Text Solution
|
- A heat engine carries one mole of an ideal monoatomic gas around the c...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- In the cyclic process shown in P-V diagram the magnitude of work done ...
Text Solution
|
- P-V plot for two gases (assuming ideal) during adiabatic processes are...
Text Solution
|
- A given mass of gas expends from the state A to the state B by three p...
Text Solution
|
- Following graph shows a single stage expansion, then work done by the ...
Text Solution
|
- One mole of an ideal monoatomic gas undergoes a cyclic process as show...
Text Solution
|
- A sample of 2 kg of helium (assumed ideal) is taken through the proces...
Text Solution
|
- Which of the following options regarding area under the graph is/are c...
Text Solution
|
- For an ideal gas subjected to different processes as shown by the grap...
Text Solution
|