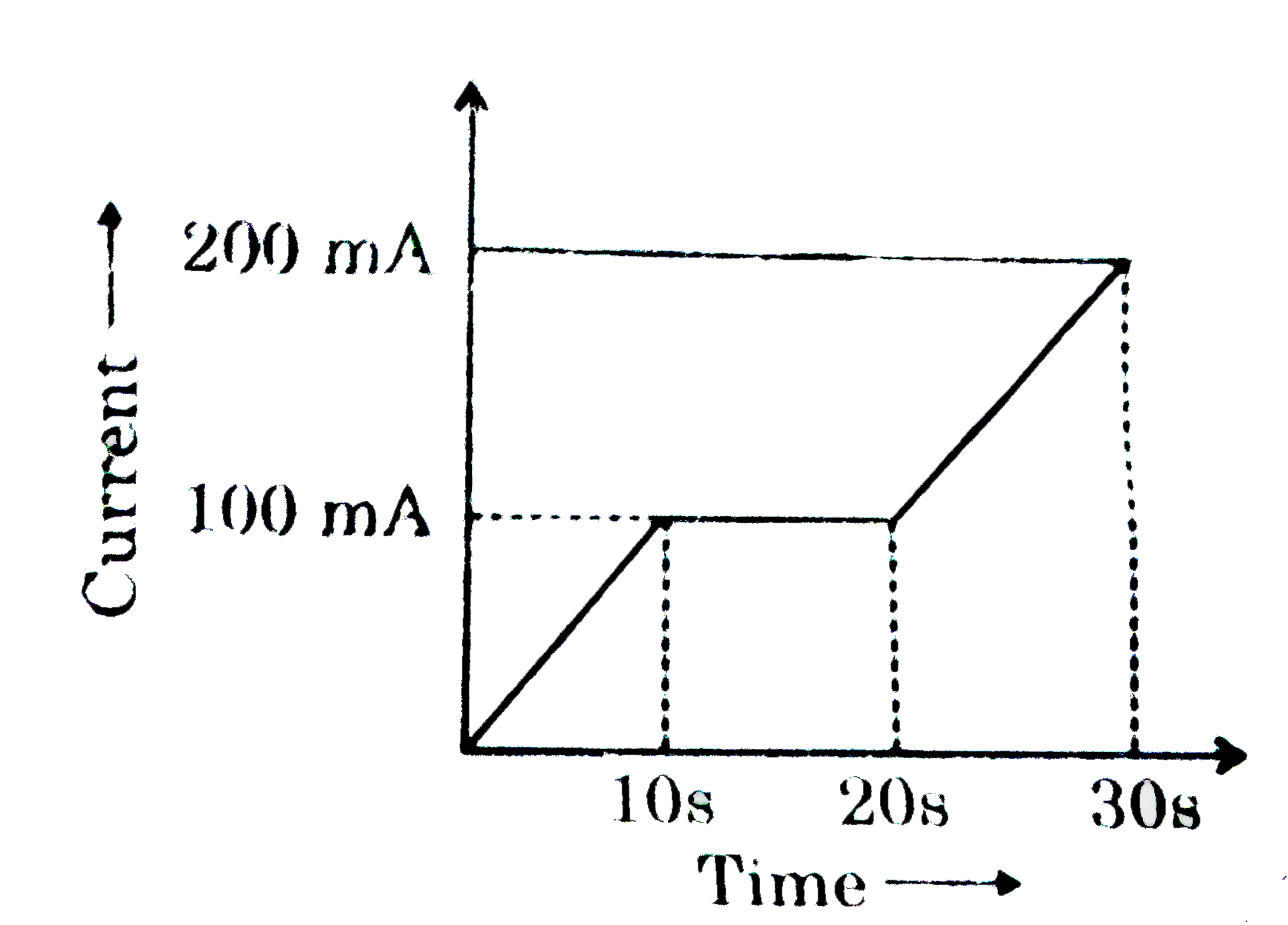A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Multiple Objective Type|68 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Comprehension 1|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise I.Electrochemistry|1 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-GRAPHICAL INTERPRETATION-Electrochemistry
- Which of the following curve represents the variation of lamda(m) with...
Text Solution
|
- Conductance measurements and be used to detect the end point of acid-b...
Text Solution
|
- Zn+Cu^(2+)(aq)toCu+Zn^(2+)(aq). Reaction quotient is Q=([Zn^(2+)])/(...
Text Solution
|
- AgNO(3) (aq) was added to an aqueous KCl solution gradually and the co...
Text Solution
|
- Electrode potential for Mg electrode varies according to the graph.
Text Solution
|
- In a Cu-voltameter, mass deposited in 30s is m gm. If the time-current...
Text Solution
|
- Which diagram best represents the change in electrical conductivity of...
Text Solution
|
- Which of the graphs shown below would best represent the changes when ...
Text Solution
|
- Select the correct option for following aqueous solution.
Text Solution
|