A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 3-CHEMISTRY
- Electrometallurgical process is used to extract
Text Solution
|
- The equivalent conductivity of 0.1 M weak acid is 100 times less than ...
Text Solution
|
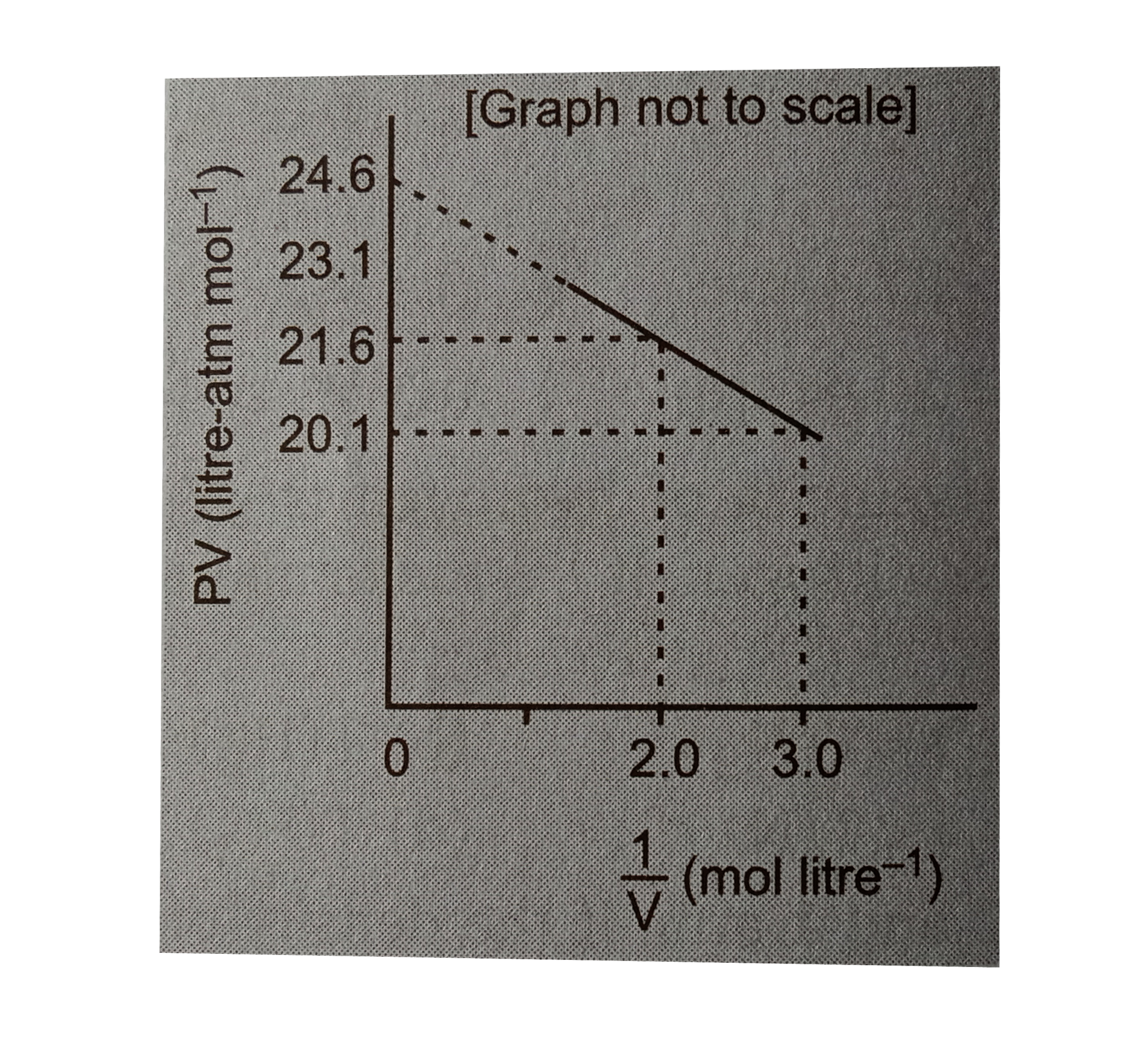
- For one mole of a van der Waals gas when b =0 and T =30 K the PV vs1//...
Text Solution
|
- An isomer of C(6)H(14) forms three monochloro derivaties. The isomer m...
Text Solution
|
- Why is Cr^(2+) reducing and Mn^(3+) oxidising when both have d^(4) con...
Text Solution
|
- The IUPAC name of the product obtained by the oxidation of phenol with...
Text Solution
|
- Three elements X, Y and Z have atomic numbers 19, 37 and 55 respective...
Text Solution
|
- In which of the following species, each atom carries same number of lo...
Text Solution
|
- An electron in an atom jumps in such a way that its kinetic energy cha...
Text Solution
|
- Which of the following compounds is not an antacid?
Text Solution
|
- How many grams of sucrose (molecular weight 342) should be dissolved i...
Text Solution
|
- 10 g of MgCO(3) decomposes on heating to 0.1 mole CO(2) and 4g MgO. Th...
Text Solution
|
- The rate of decomposition for methyl nitrite and ethyl nitrite can be ...
Text Solution
|
- A body centred cubic lattice is made up of hollow sphere of B. Sphere ...
Text Solution
|
- The equilibrium constant for CN^(-) + CH(3)COOH hArr HCN + CH(3)COO^...
Text Solution
|
- Which of the following is a cyclic oxoacid
Text Solution
|
- Among given compounds, how many compounds will react with NaHCO(3) or ...
Text Solution
|
- The critical micelle concentration (CMC) of a cationic colloidal elect...
Text Solution
|
- The ammonia evolved from the treatment of 0.30 g of an organic compoun...
Text Solution
|
- The ratio K(p) to K( c) of a reaction is 24.63 L atm mol^(-1) at 27^(@...
Text Solution
|
 .
.