Topper's Solved these Questions
ELECTRONS AND PHOTONS
SL ARORA|Exercise Problem Solution|19 VideosELECTRONS AND PHOTONS
SL ARORA|Exercise TYPE A : Problem For Self Practice|21 VideosELECTROMAGNETIC WAVES
SL ARORA|Exercise Problem for self-practice|21 VideosELECTROSTATIC FORCES, CHARGES AND FIELDS
SL ARORA|Exercise Problems For Self Practice|1 Videos
Similar Questions
Explore conceptually related problems
SL ARORA-ELECTRONS AND PHOTONS-TYPE E : Problem For Self Practice
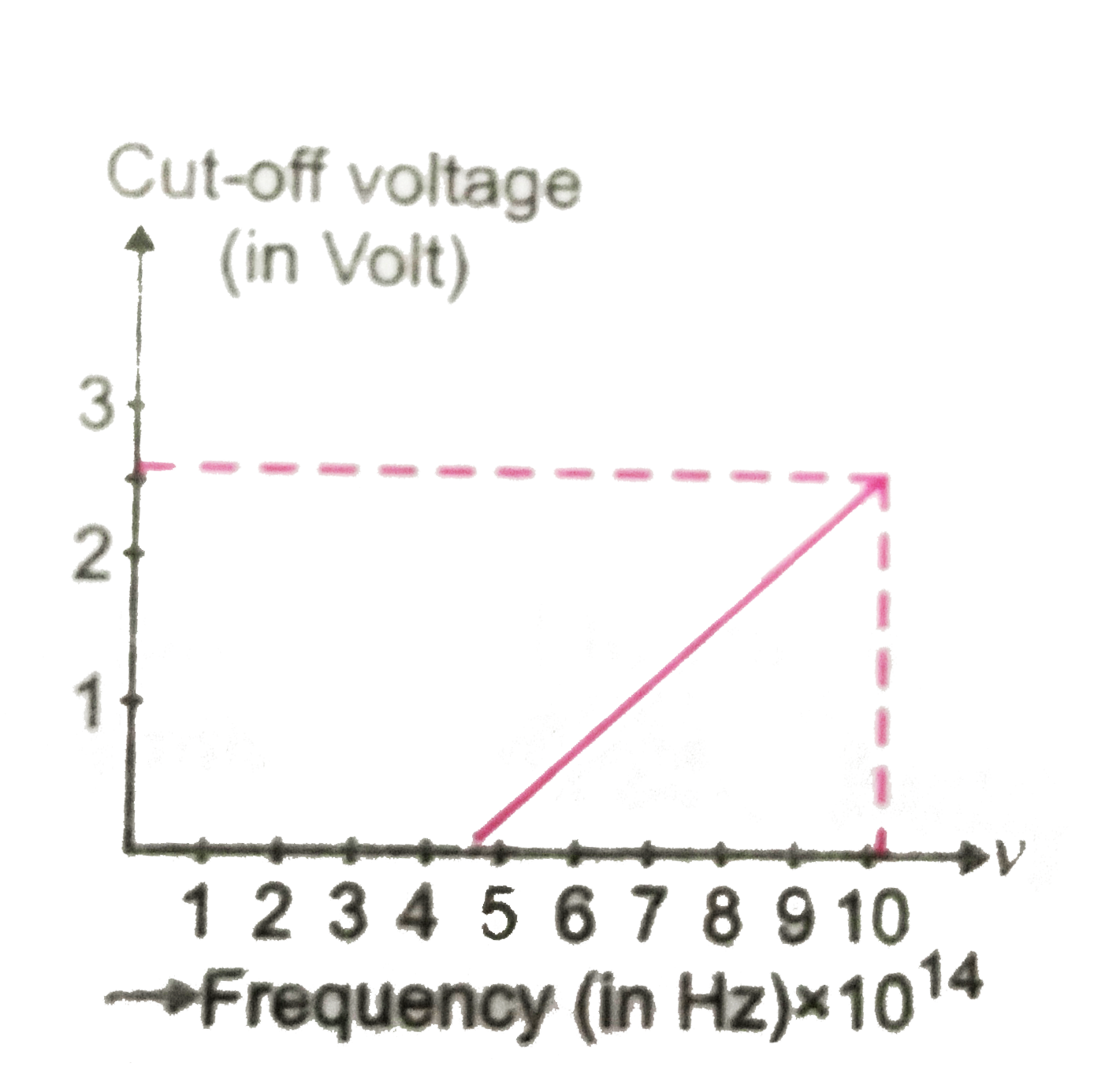
- For photoelectric effect in sodium, fig. shows the plot of cut-off vol...
Text Solution
|
- Calculate the de Broglie wavelength associated with the following : ...
Text Solution
|
- Calculate the de-Broglie wavelength in nm associated with a ball of ma...
Text Solution
|
- Calculate the de-Broglie wavelength associated with an alpha-particle ...
Text Solution
|
- Calculate the velocity of a neutron having de Broglie wavelength of 10...
Text Solution
|
- Calculate the de-Broglie wavelength of an electron of kinetic energy 1...
Text Solution
|
- For what kinetic energy of a neutron will the associated de-Broglie wa...
Text Solution
|
- The de-Broglie wavelength of an electron is 4 Å. What is its momentum ...
Text Solution
|
- What voltage must be applied to an electron microscope to produce elec...
Text Solution
|
- Energy of a particle at absolute temperature T is of the order kT. Cal...
Text Solution
|
- The angle of reflection for first order monochromatic X-rays from a cr...
Text Solution
|
- Monochromatic X-rays, when reflected from a crystal with lattice spaci...
Text Solution
|