Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 4-PHYSICS - SUBJECTIVE NUMERICAL
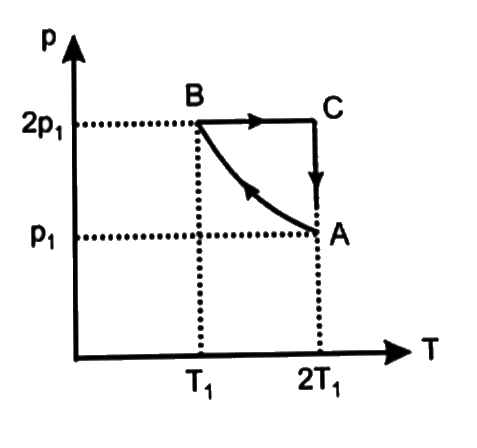
- Two moles of an ideal monoatomic gas is taken through a cycle ABCA as ...
Text Solution
|
- A uniform rod of mass M(1) is hinged at its upper end as shown in the ...
Text Solution
|
- In the circuit shown in the figure, the switch was kept in position - ...
Text Solution
|
- Three particle each of mass m, are located at the vertices of an equil...
Text Solution
|
- In the given arrangement, the rod is free to rotate about the hinge an...
Text Solution
|