A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 4-CHEMISTRY - SUBJECTIVE NUMERICAL
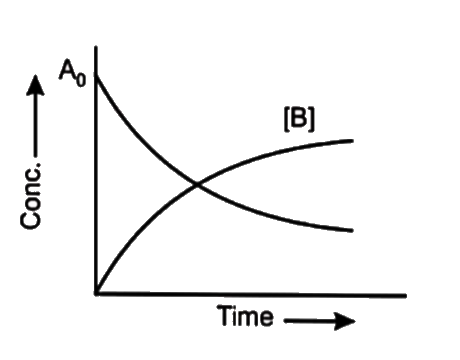
- At the point of intersection of the two curves shown the concentration...
Text Solution
|
- Bromine in excess is dropped to a 0.01 M SO(2). All of SO(2) is oxidiz...
Text Solution
|
- Among the following, the total number of componds containing at least ...
Text Solution
|
- Species like SbCl(6)^(-), SnCl(6)^(2-), XeF(5)^(+) and IO(6)^(5-) has ...
Text Solution
|
- Find out the double bond equivalent (DBE) value of the given following...
Text Solution
|
- 2.68xx10^(-3) moles of solution containing anion A^(n+) require 1.61xx...
Text Solution
|