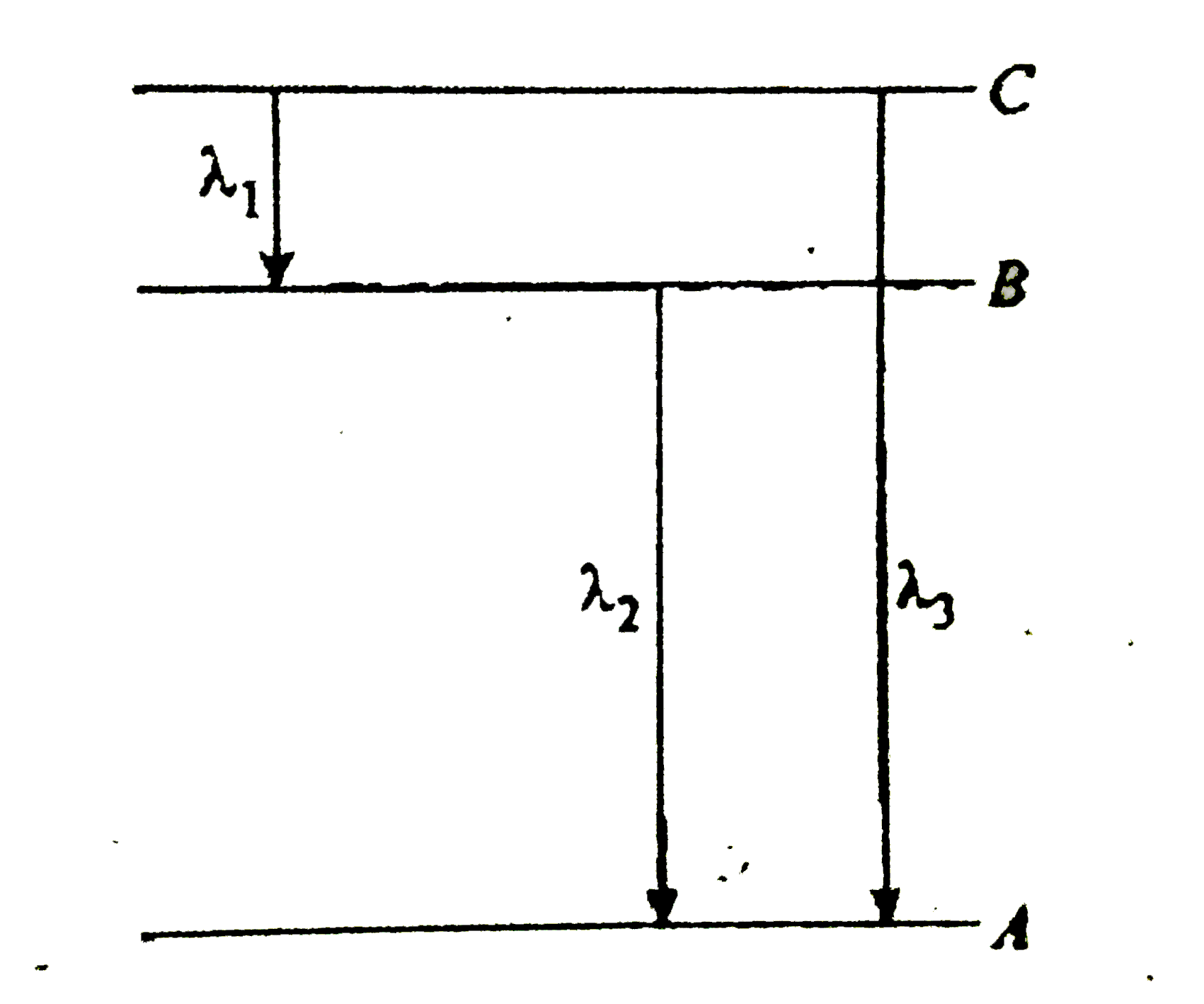
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC PHYSICS
PHYSICS GALAXY - ASHISH ARORA|Exercise NumericalMCQsSingle OptiorisCorrect|49 VideosATOMIC PHYSICS
PHYSICS GALAXY - ASHISH ARORA|Exercise Advance MCQs with One or More Options Correct|30 VideosATOMIC PHYSICS
PHYSICS GALAXY - ASHISH ARORA|Exercise Discussion Question|17 VideosCAPACITANCE
PHYSICS GALAXY - ASHISH ARORA|Exercise UNSOLVED NUMERICAL PROBLEMS|40 Videos
Similar Questions
Explore conceptually related problems
PHYSICS GALAXY - ASHISH ARORA-ATOMIC PHYSICS-Conceptual MCQs Single Option Correct
- In the Bohr model of a hydrogen atom, the centripetal force is furnish...
Text Solution
|
- If elements with principal quantum number ngt 4 were not allowed in na...
Text Solution
|
- Bohr's atomic model gained acceptance above all other models because i...
Text Solution
|
- Pauli's exclusion principle states that'no&vo electrons in an atom can...
Text Solution
|
- Energy levels A,B and C of a certain atom correspond to increasing val...
Text Solution
|
- When white light (violet to red) is passed through hydrogen gas at roo...
Text Solution
|
- The difference in angular momentum associated with electron in two suc...
Text Solution
|
- If radiation of all wavelengths from ultraviolet to infrared ispassed ...
Text Solution
|
- Which of the following force is responsible for alpha-particle scatter...
Text Solution
|
- A Hydrogen atom and Li^(++) ion are both in the second excited state....
Text Solution
|
- The minimum kinetic energy of an electron,hydrogen ion,helium ion requ...
Text Solution
|
- The wavelength of radiation emitted due to transition of electron from...
Text Solution
|
- A neutron collies head-on with a stationary hydrogen atom in ground st...
Text Solution
|
- An electron in hydrogen atom after absorbing-an energy photon jumps fr...
Text Solution
|
- Mark correct statements:
Text Solution
|
- Figure represents transitions of electrons from higher to lower state ...
Text Solution
|
- Hydrogen H, deuterium D, singlyionized helium He^(+) and doubly ionize...
Text Solution
|
- Which ofthe following curves may represent the energy of electron in h...
Text Solution
|
- A hydrogen atom in ground state absorbs 12.1 eV energy. The orbital an...
Text Solution
|
- Magnetic moment due to the motion of the electron in n^(th) energy sta...
Text Solution
|