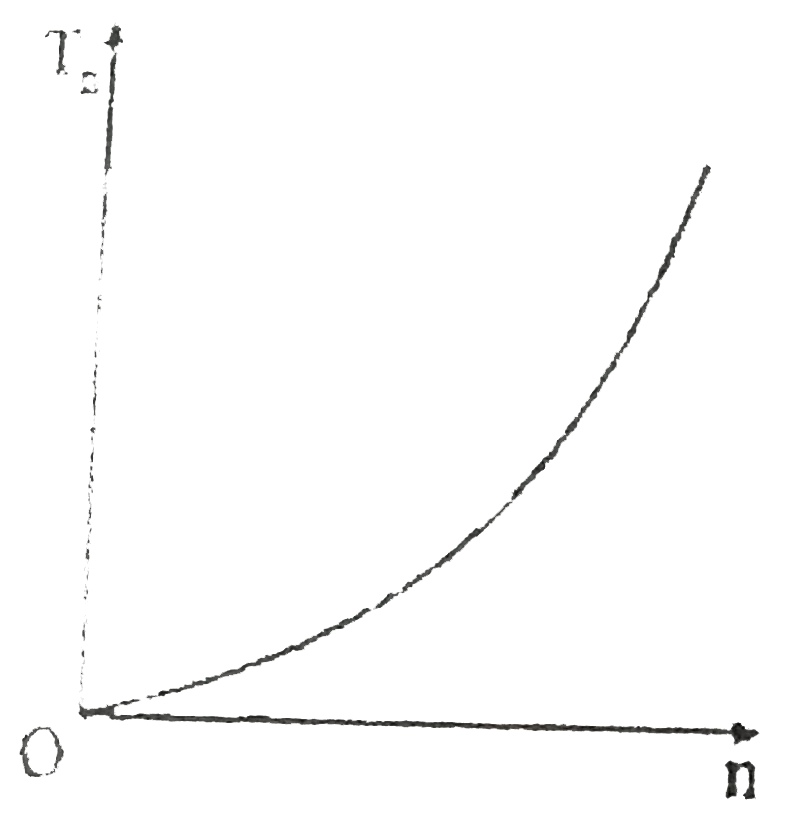
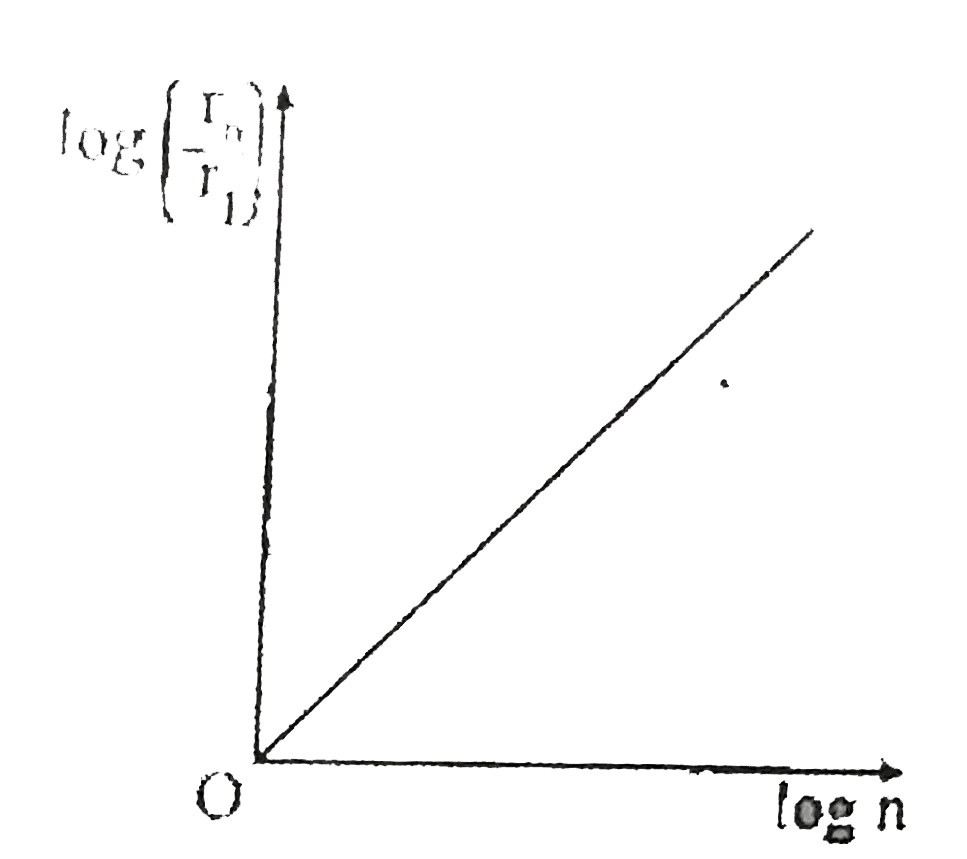
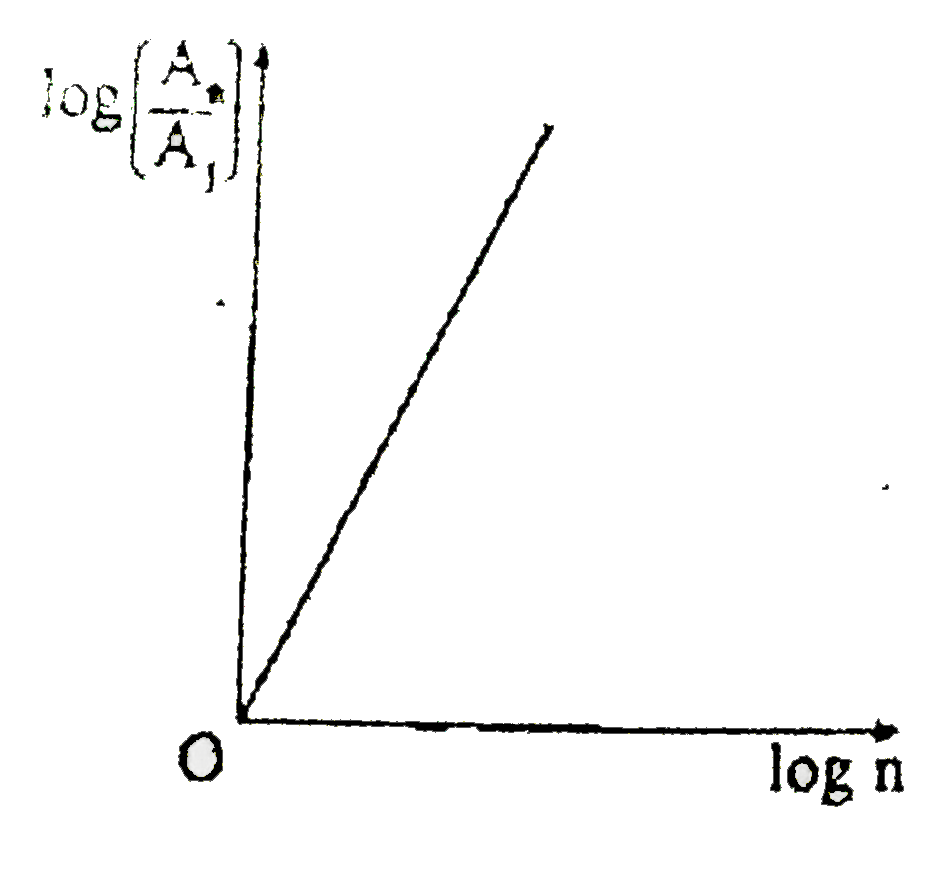
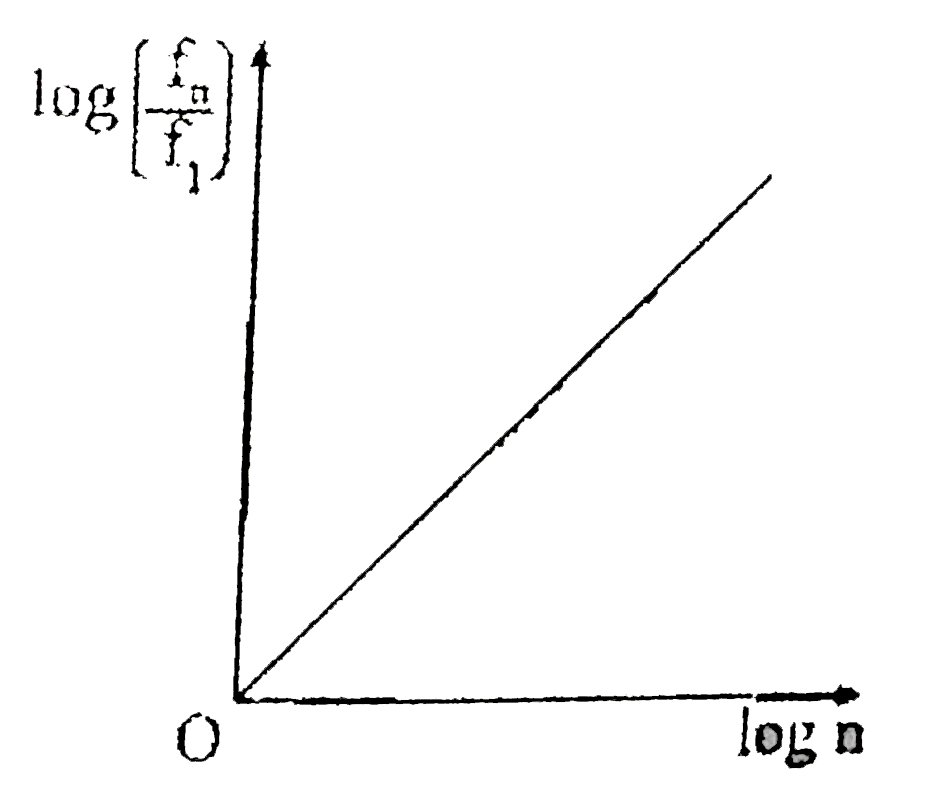
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS GALAXY - ASHISH ARORA-ATOMIC PHYSICS-Advance MCQs with One or More Options Correct
- Whenever a hydrogen atom emits a photon in the Balmer series .
Text Solution
|
- Which of the following statements about hydrogen spectrum are correct?
Text Solution
|
- A neutron collies head-on with a stationary hydrogen atom in ground st...
Text Solution
|
- If, in a hydrogen atom, radius of nth Bohr orbit is r(n) frequency of ...
Text Solution
|
- Mark correct statements(s):
Text Solution
|
- A photon of energy 10.5eV is allowed to interact with a hydrogen atom ...
Text Solution
|
- When a hydrogen atom is excited from ground state to first excited s...
Text Solution
|
- Suppose the potential energy between an electron and a proton at a dis...
Text Solution
|
- An electron in an hydrogen atom has total energy of -3.4eV. Choose the...
Text Solution
|
- when Z is doubled in an atom, which of the following statements are co...
Text Solution
|
- The electron in a hydrogen atom jumps back from an excited state to gr...
Text Solution
|
- the photon radiated from hydrogen corresponding to the second line of ...
Text Solution
|
- A particular hydrogen like atom has its ground state Binding energy = ...
Text Solution
|
- If radiation of all wavelengths from ultraviolet to infrared ispassed ...
Text Solution
|
- In the hydrogen atom, if the reference level of potential energy is as...
Text Solution
|
- Choose the correct statement (s) for hydrogen and deuterium atoms (con...
Text Solution
|
- A neutron collies head-on with a stationary hydrogen atom in ground st...
Text Solution
|
- The figure shows an energy level diagram for the hydrogen atom. Severa...
Text Solution
|
- A hydrogen atom in the 4th excited state, then:
Text Solution
|
- An electron with kinetic energy E collides with a hydrogen atom in the...
Text Solution
|