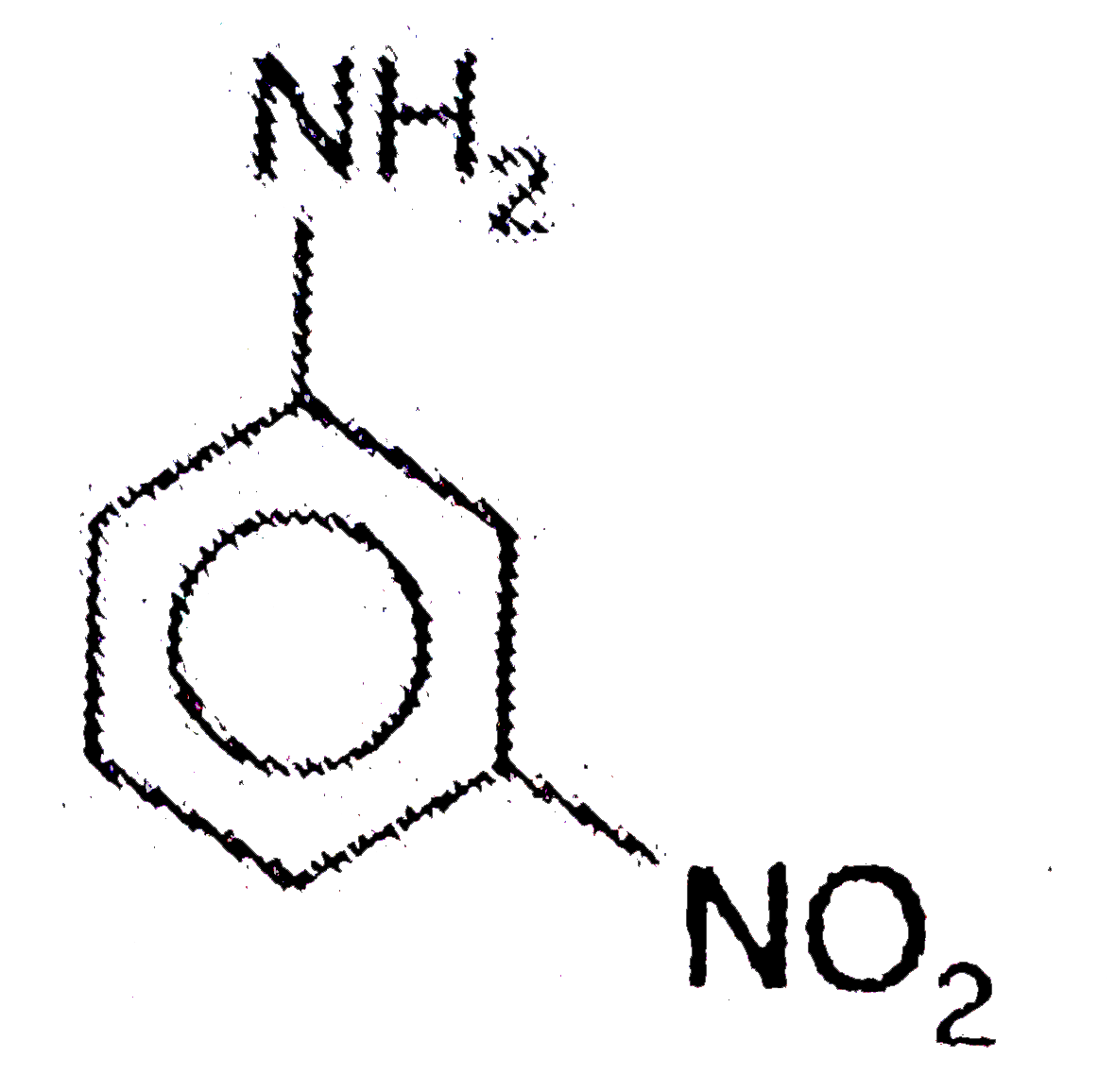
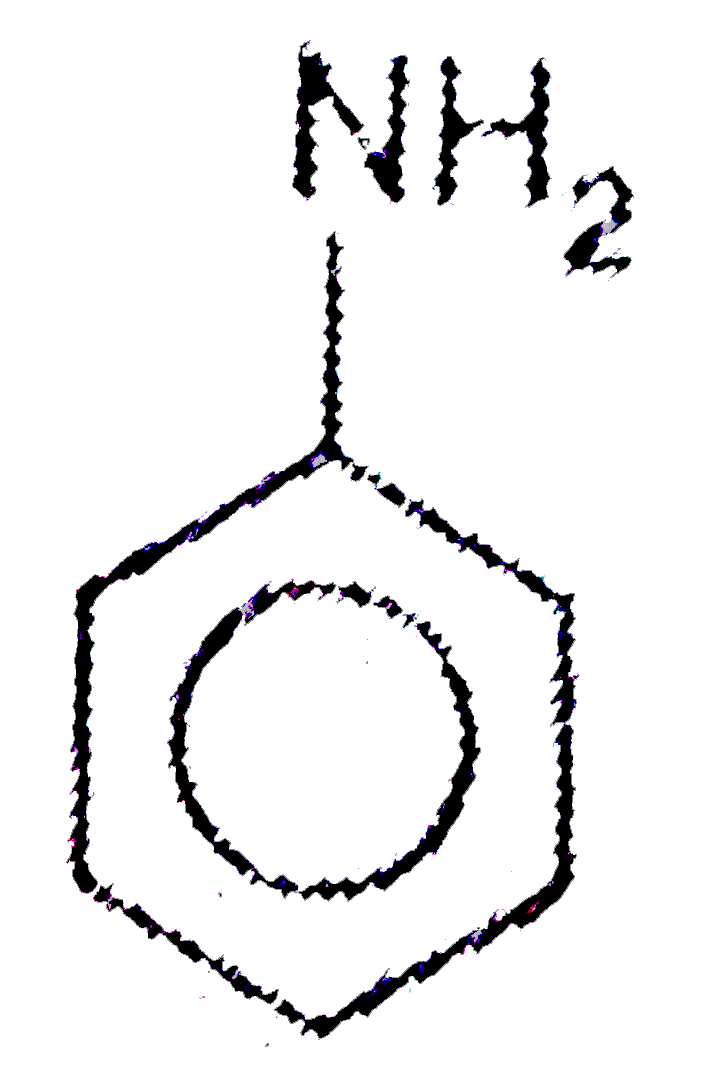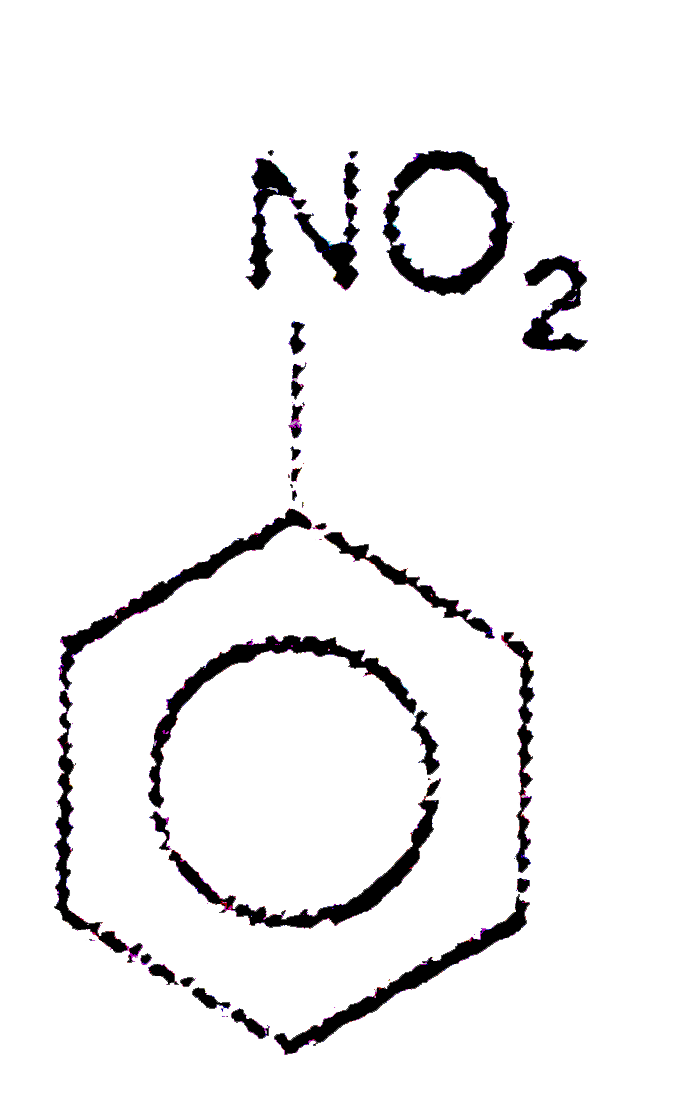A
B
C
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-CHEMICAL BONDING-BITSAT Archives
- Which of the following will have largest dipole moment?
Text Solution
|
- Which of the following pairs has identical shape?
Text Solution
|
- Using MOT, which of the following pairs denote paramagnetic species?
Text Solution
|
- Which of the following is isoelectronic with carbon?
Text Solution
|
- Which of the following does not contain a coordinate bond?
Text Solution
|
- The number of unpaired electrons in nickel carbonyl, is
Text Solution
|
- The highest bond strength is shown by
Text Solution
|
- Which of the following has maximum dipole moment?
Text Solution
|
- Which one of the following species has the largest internuclear distan...
Text Solution
|
- The pair of species with similar shape is
Text Solution
|
- The bond length of HCl molecule is 1.275 Å and its dipole moment is 1....
Text Solution
|
- Which of the following is a correct set ?
Text Solution
|
- The isoelectronic pair is
Text Solution
|
- Which of the following species has a bond order other than 3?
Text Solution
|
- The molecular electronic configuration of Be(2) is
Text Solution
|