A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-REDOX REACTIONS-Bitsat Archives
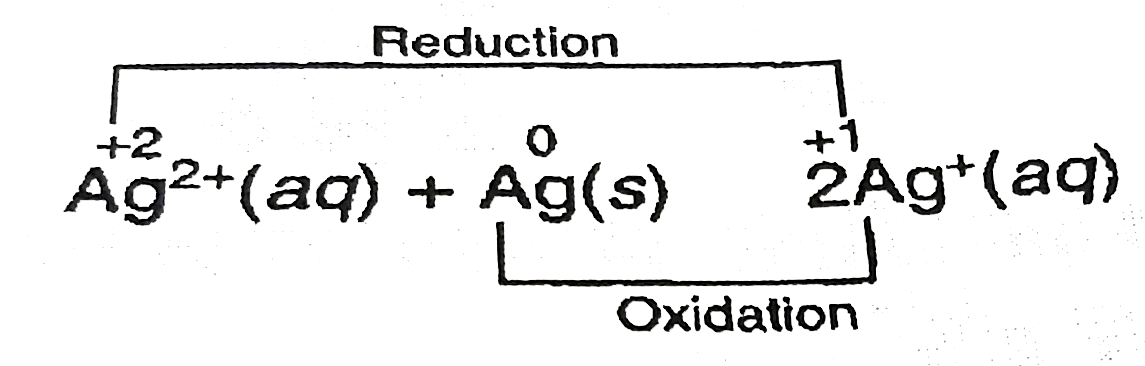
- Following reaction is an example of Ag^(2+) (aq) + Ag (s) hArr 2Ag^...
Text Solution
|
- The ratio of oxidation states of Cl in potassium chloride to that in p...
Text Solution
|
- The oxidation number of sulphur in Na(2)S(4)O(6) is
Text Solution
|
- 2MnO(4)^(-) + 5H(2)O(2) + 6H^(+) rarr 2Z + 5O(2) + 8H(2)O Identify Z i...
Text Solution
|
- The oxidation number of N and Cl in NOClO(4) respectively are
Text Solution
|
- Which one of the following reactions represent the oxidising properly ...
Text Solution
|
- The speicies that undergoes disproportionation in an alkaline medium i...
Text Solution
|
- A compound contains X, Y and Z atoms. The oxidation state of X, Y and ...
Text Solution
|
- The oxidation number of oxygen in hydrogen peroxide is
Text Solution
|