A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-HYDROGEN-BITSAT Archives
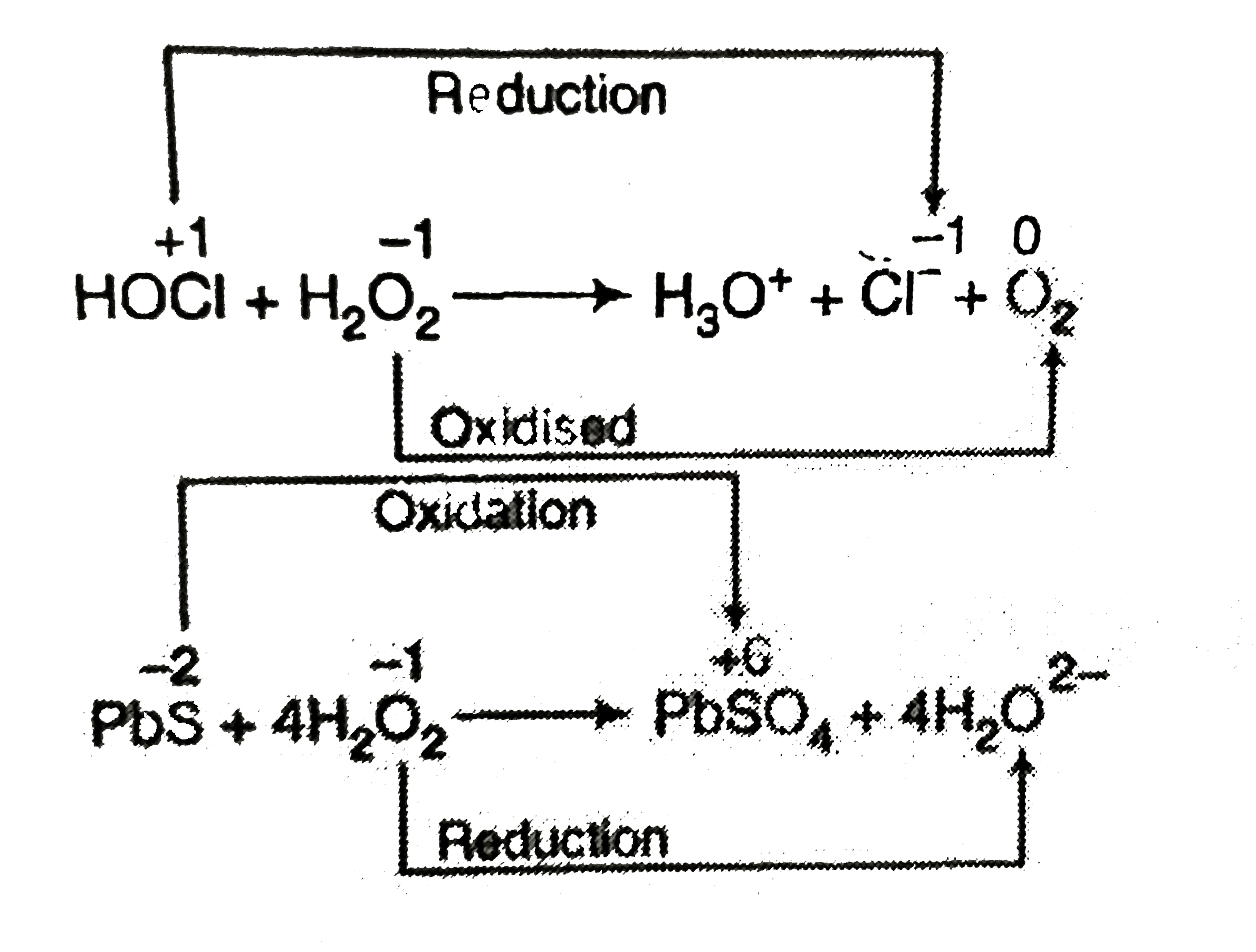
- Study the following reaction carefully I. HOCl+ H(2)O(2) to H(3)O...
Text Solution
|
- Which one the following is a covalent hydride ?
Text Solution
|
- What is formed when calcium carbide reacts with heavy water?
Text Solution
|
- H(2)O(2) is always stored in black bottles because
Text Solution
|
- H(2)O(2) used in rockets has the concentration
Text Solution
|
- Calgon used as water softner is
Text Solution
|