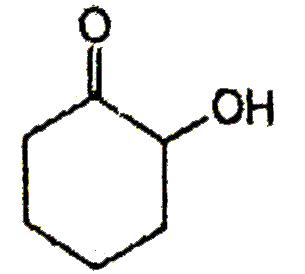
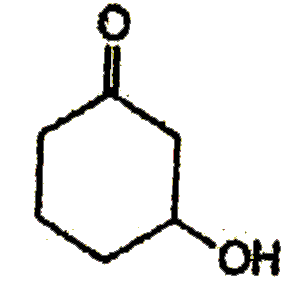
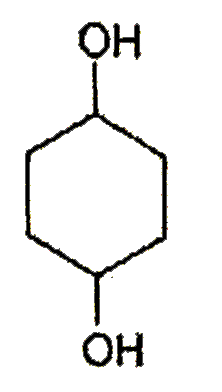
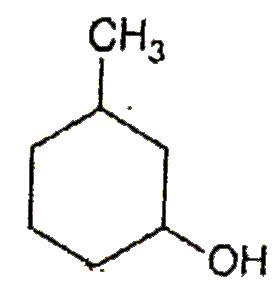
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-ALDEHYDE AND KETONES-Bitsat Archives
- Maximum dehydration takes place in that of
Text Solution
|
- How many chiral centres are possible for the product of following reac...
Text Solution
|
- Arrange the following compounds in the increasing order of nucleophill...
Text Solution
|
- Which of the following compounds will give positive iodoform test with...
Text Solution
|
- What will be the final product of the reaction?
Text Solution
|
- The compound formed as a result of oxidation of propyl benzene by KMnO...
Text Solution
|
- What will be the correct structural formula of product for the followi...
Text Solution
|
- Which of the following is process used for the preparation of acetone?
Text Solution
|
- What will be the main product when acetylene reacts with hypochlorous ...
Text Solution
|
- Which of the following reagents can be used to prepare benzaldehyde fr...
Text Solution
|
- Acetone on addition to methyl magnesium bromide froms a complex, which...
Text Solution
|
- CH(3)C-=C CH(3) overset((i)X) underset((ii)H(2)O //Zn)rarr X in the...
Text Solution
|
- Which of the following ketones will not respond to iodoform test?
Text Solution
|
- Acetone and acetaldehyde can be distinguished by
Text Solution
|
- Cyanohydrin of which of the following forms lactic acid
Text Solution
|