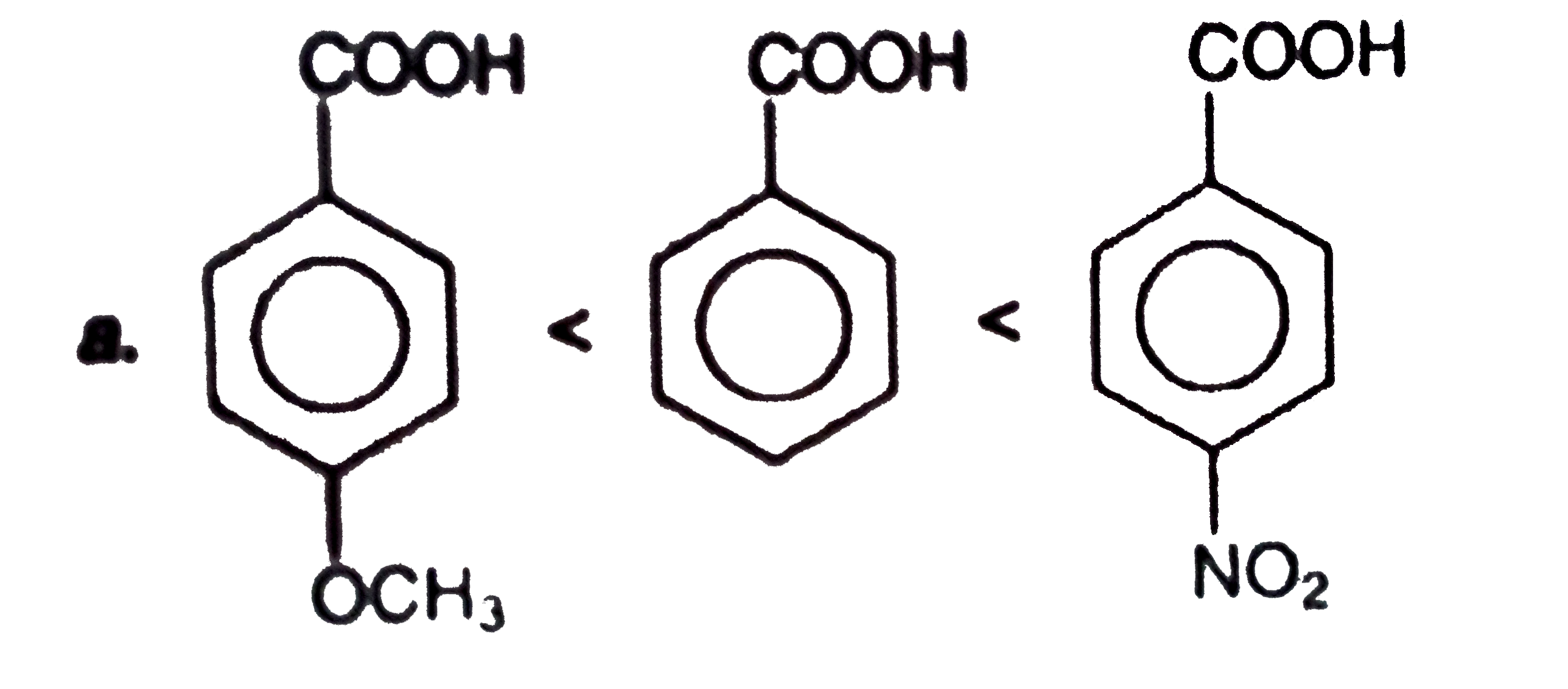
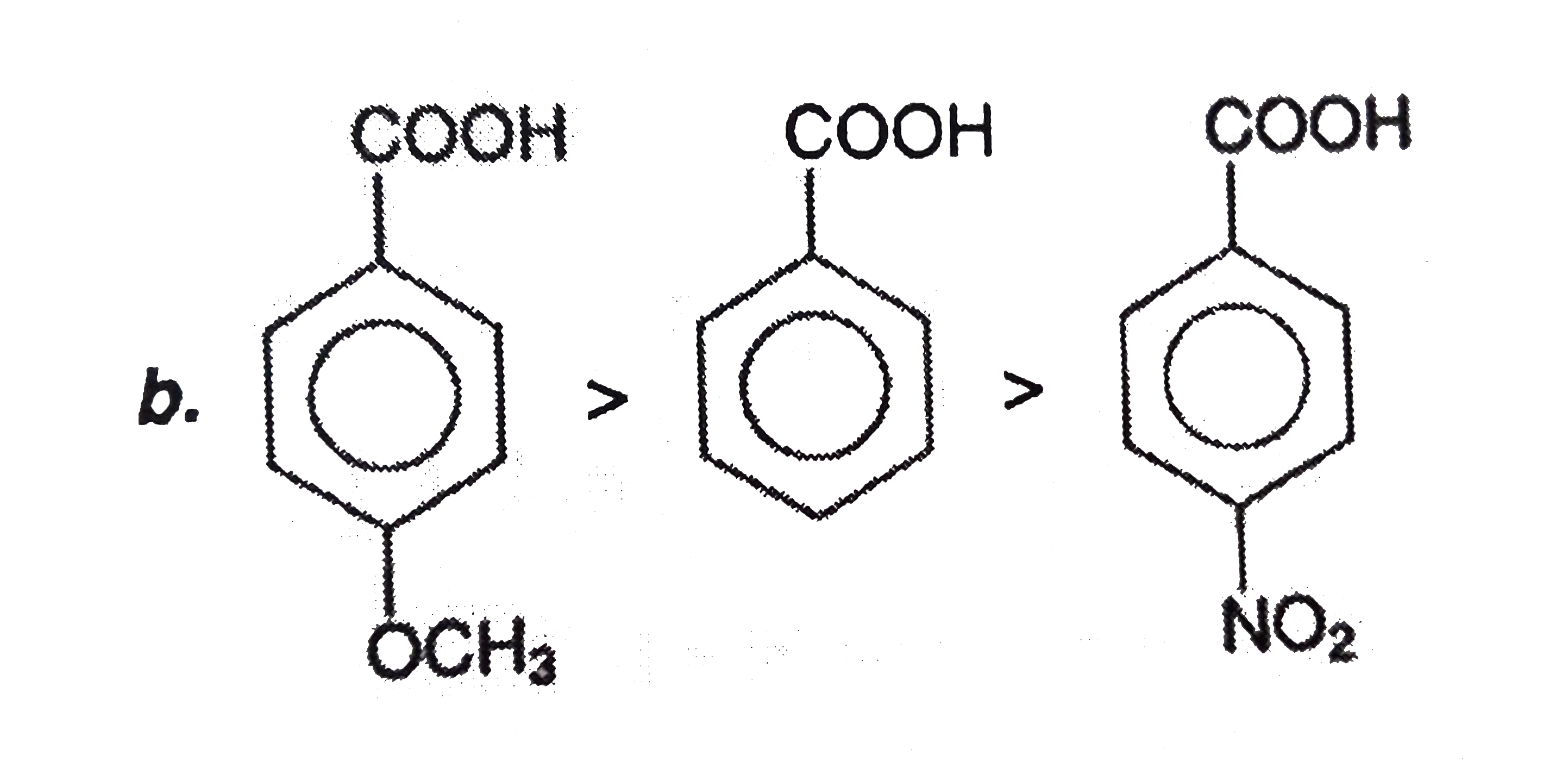
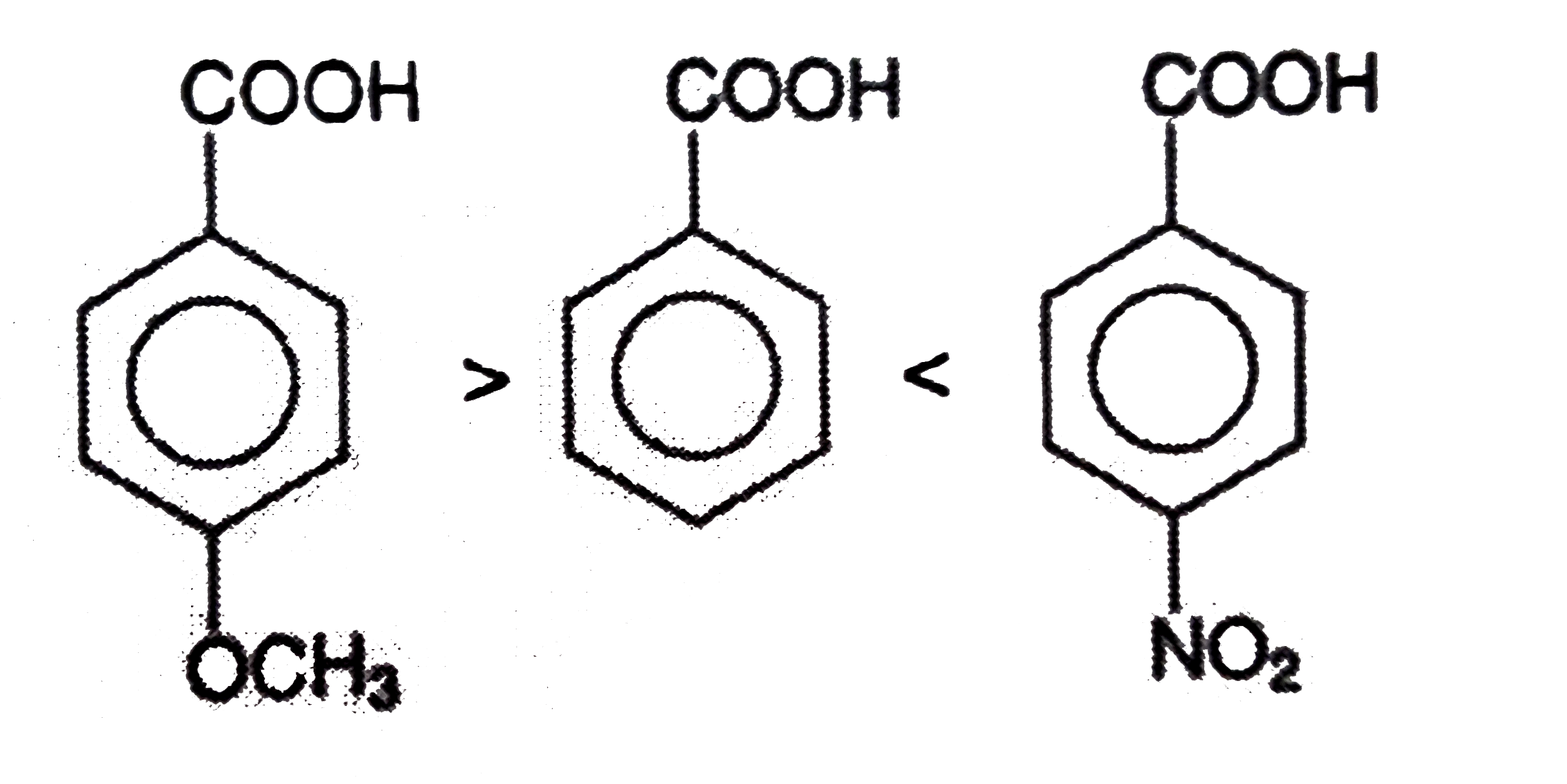
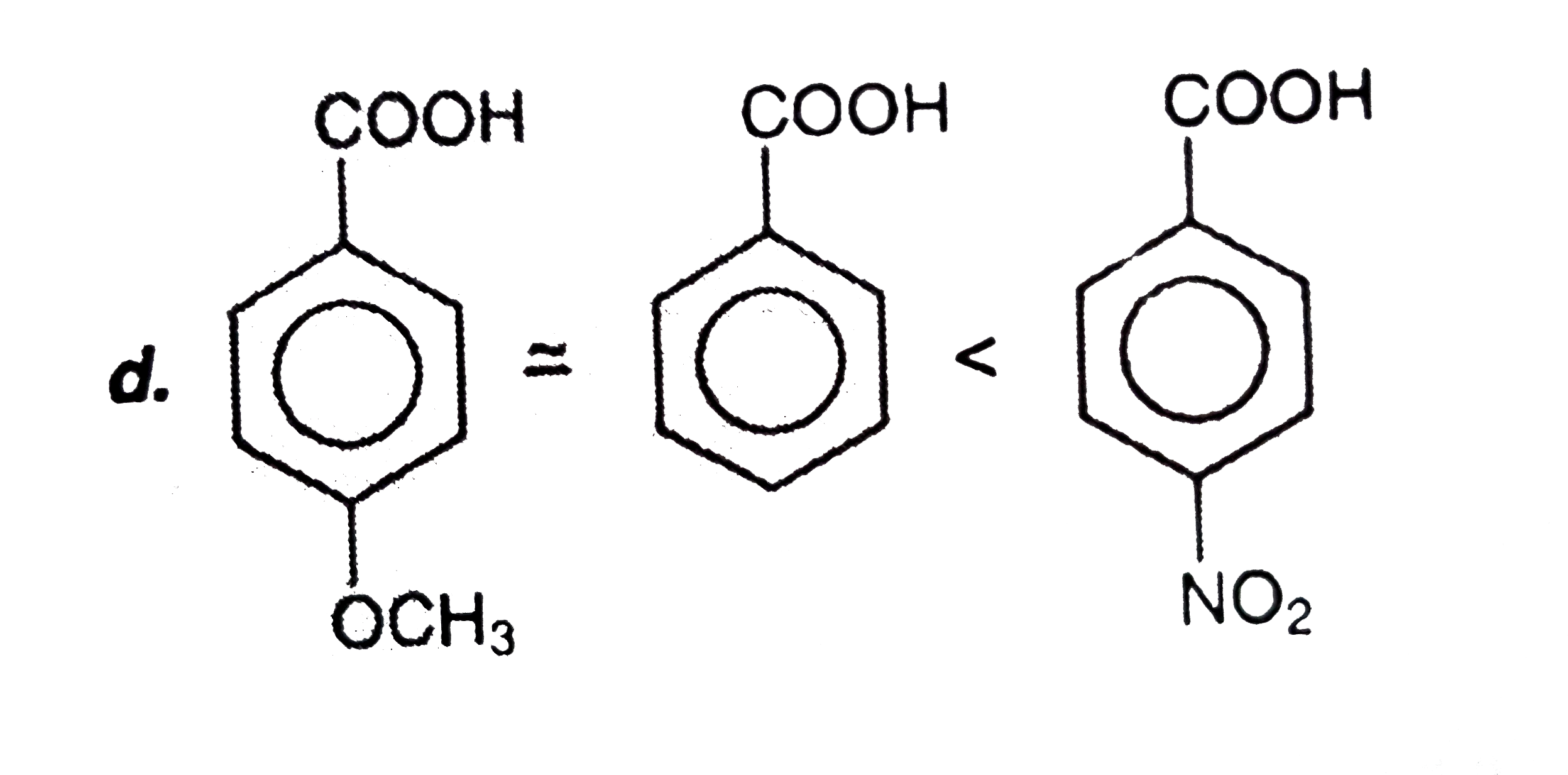
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-CARBOXYLIC ACID AND DERIVATIVES-All Questions
- The correct order for the acidic strength of thhe following compounds ...
Text Solution
|
- Consider the following reaction. CH(2)=CHCH(2)Br overset(Mg//"dry et...
Text Solution
|
- Arrange the following derivative of carboxylic acid in their increasin...
Text Solution
|
- Select the missing compounds for the given reaction Identify A,B ...
Text Solution
|
- Which of the following represents the correct order of the acidic stre...
Text Solution
|
- Major product of the above reaction is
Text Solution
|
- To distinguish between formic acid and acetic acid, the suitable reage...
Text Solution
|
- When salicylic acid is heated with acetic anhydride, we get
Text Solution
|
- The general formula of the compound obtained as a result of reaction b...
Text Solution
|
- Pyruvic acid as
Text Solution
|
- The product of which of the following reaction is capable of changing ...
Text Solution
|
- The compound that undergoes decarboxylation most readily under mild co...
Text Solution
|
- The acids, HCOOH and CH(3)COOH are distinguished by
Text Solution
|
- The reaction given below is called CH(3)CH(2)CH(2)COOHoverset"Red" P...
Text Solution
|
- The product formed during Hell-Volhard-Zelinsky reaction is
Text Solution
|
- In the given reactions: Identify X and Y for the above reaction,
Text Solution
|
- Complete the following reaction
Text Solution
|
- In a set of the given reactions, acetic acid yields a product C. CH(...
Text Solution
|
- Complete the synthesis by giving the missing product.
Text Solution
|
- Role of 2,4-dichlorophenoxy acetic acid is used as
Text Solution
|