A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level -I (H.W)
- 5 mol of gas at 5 atmospheric pressure contained in a 100 L cylinder a...
Text Solution
|
- Frictionless and weightless piston was fitted into a cylinder containi...
Text Solution
|
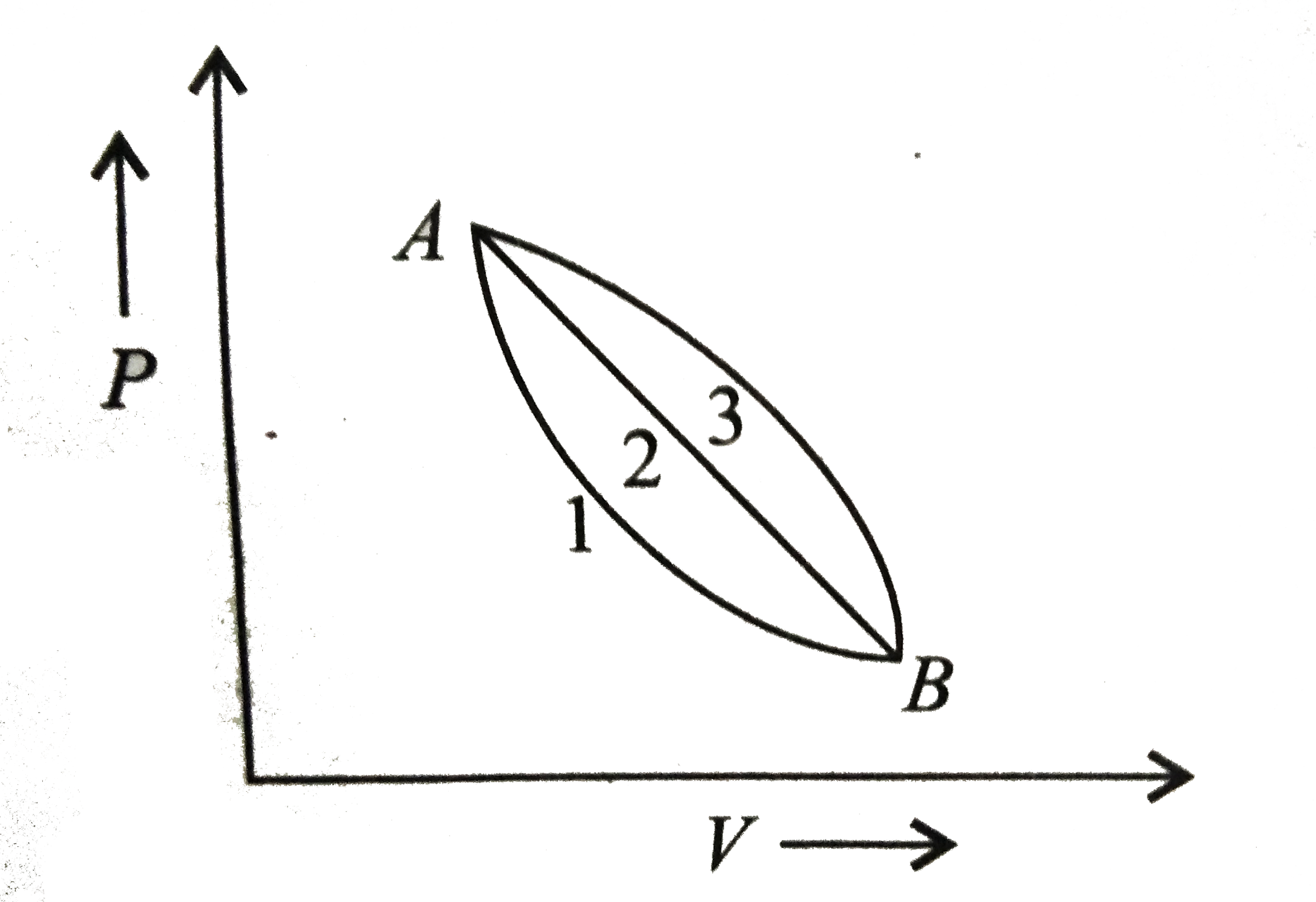
- A given mass of gas expands from state A to state B by three paths 1,2...
Text Solution
|
- One mole of liquid water at its boiling point vaporises against a cons...
Text Solution
|
- Heat capacity of water is 18 cal-"degree"^(-1)-mol^(-1). The quantity ...
Text Solution
|
- Heat capacity (C(V)) of an ideal gas is X KJ/mole/K. To rise its tempe...
Text Solution
|
- The relationship between Delta H and Delta E for the reaction PCl(3)(g...
Text Solution
|
- If Delta E is the heat of reaction for C(2)H(5)OH((1))+3O(2(g)) rarr 2...
Text Solution
|
- For the system S (s) + O(2)(g) rarr SO(2)(g) ?
Text Solution
|
- In the complete combustion of butanol C(4)H(9)OH(l), if Delta H is ent...
Text Solution
|
- Which one of the following is an endothermic reaction ?
Text Solution
|
- Which of the following is an exothermic reaction?
Text Solution
|
- The heats evolved in combustion of rhombic and monoclinic sulphur are,...
Text Solution
|
- Conversion of sulphur to SO(3) has Delta H = -2x Kcal and conversion o...
Text Solution
|
- In which of the following reactions, heat liberated is known as standa...
Text Solution
|
- In which of the following reaction does the heat change represent the ...
Text Solution
|
- For the reaction 3N(2)O((g))+2NH(3(g)) rarr 4N(2(g))+3H(2)O((g))Delt...
Text Solution
|
- In order to decompose 9 grams of water 142.5 KJ heat is required. Henc...
Text Solution
|
- The standard heat of formation of carbon disulphide (l) given that sta...
Text Solution
|
- H(2)(g) + Cl(2) rarr 2HCl (g), Delta H = -44K.cals 2 Na(s)+2HCl (g)rar...
Text Solution
|