A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level -V
- Choose the correct statements:
Text Solution
|
- 200g of water is contained in a beaker of mass 150 f at 20^(@)C. The t...
Text Solution
|
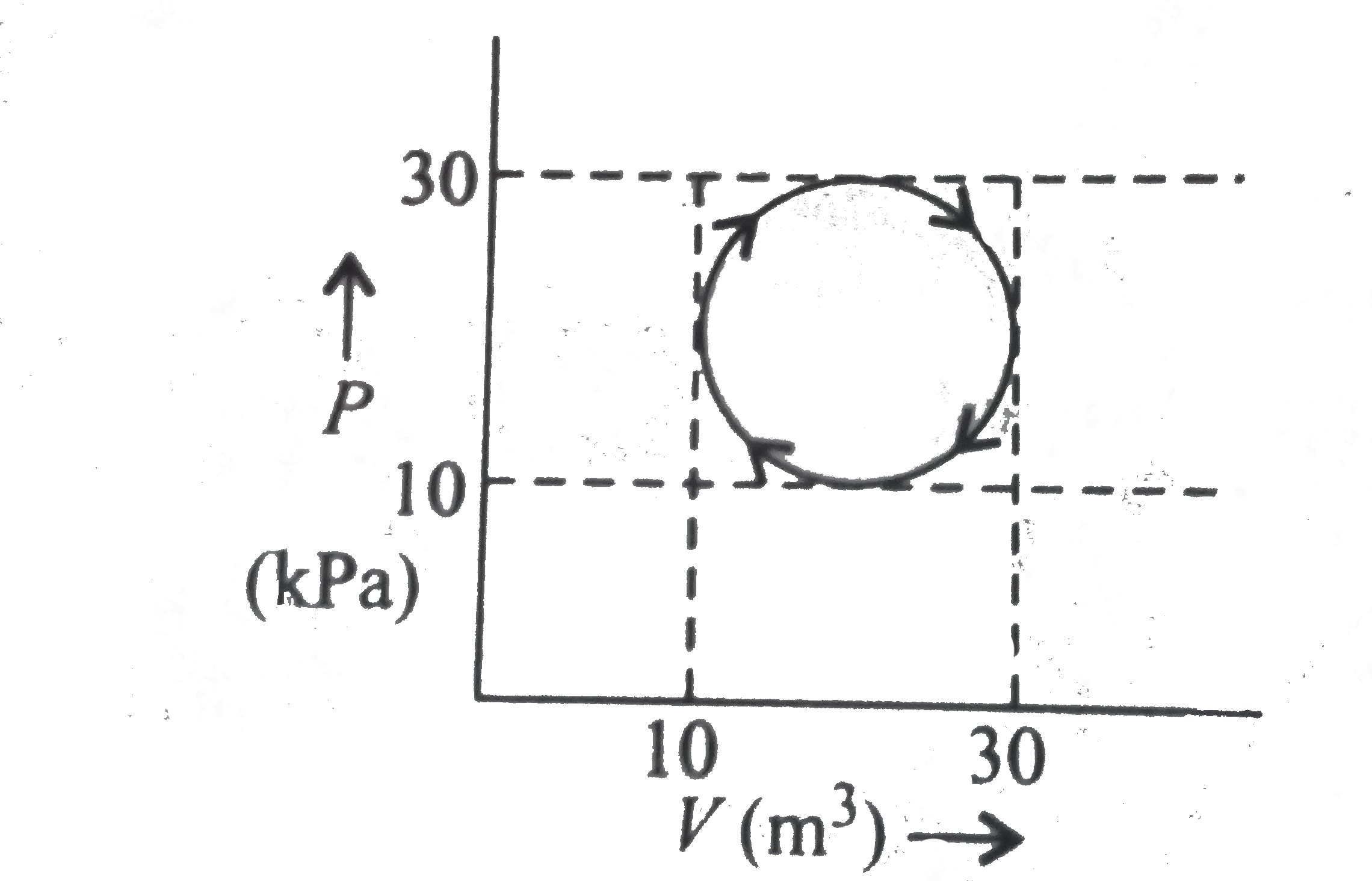
- Which one is not correct for a cyclic process as shown in the figure ?
Text Solution
|
- Select the incorrect statements for the equillibrium under standard co...
Text Solution
|
- Given, 2Fe2O(3(s)) to 4Fe((s)) + 3 O(2(g)), Deltar G1^@ = +1487kJmol^(...
Text Solution
|
- Which of the following is correct for the change shown below ? under...
Text Solution
|
- Which of the following graphs given below show (s) adiabatic process?
Text Solution
|
- He,N(2), and O(3) are expanded adiabatically and their expansion curve...
Text Solution
|
- Heat of neutralisation of strong acid and strong base under 1 atm and ...
Text Solution
|
- One mole of CH(3)COOH undergo dimerization in vapour state at 127^(@)C...
Text Solution
|
- Work done in expansion of an idela gas from 4L to 6L against a constan...
Text Solution
|
- What work is to be done on 2mol of a perfect gas at 27^(@)C it is comp...
Text Solution
|
- If one mole of an ideal gas with C(v) = (3)/(2) R is heated at a const...
Text Solution
|
- One mole of an ideal gas is subjected to a two step reversible process...
Text Solution
|
- The normal boiling point of a liquid X is 400 K. Which of the fol...
Text Solution
|
- From the following data, mark the option (s) where Delta H is correctl...
Text Solution
|
- The bond dissociation energy of a diatomic molecule is also called bon...
Text Solution
|
- The bond dissociation energy of a diatomic molecule is also called bon...
Text Solution
|
- The bond dissociation energy of a diatomic molecule is also called bon...
Text Solution
|
- The first law thermodynamics was gives as q=Delta U+(-w), where q is h...
Text Solution
|