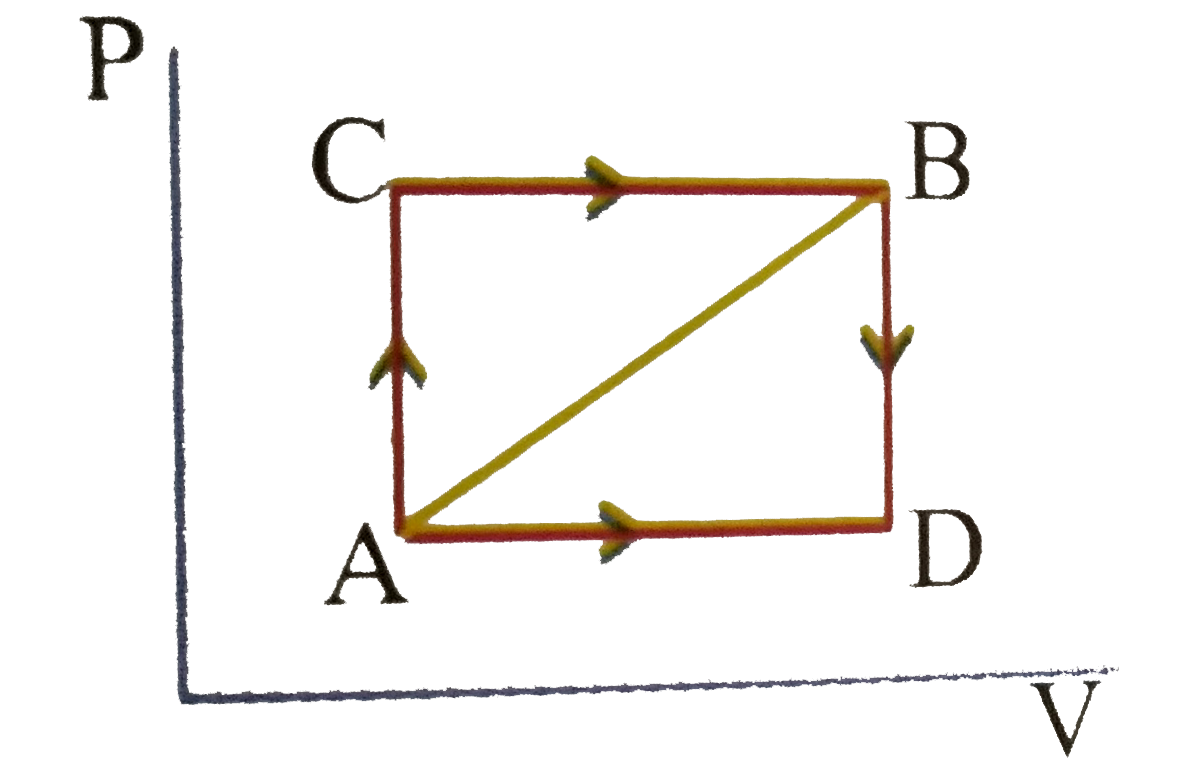
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level -V
- The bond dissociation energy of a diatomic molecule is also called bon...
Text Solution
|
- The first law thermodynamics was gives as q=Delta U+(-w), where q is h...
Text Solution
|
- The first law thermodynamics was gives as q=Delta U+(-w), where q is h...
Text Solution
|
- The first law of thermodynamics was given as q=DeltaU+(-w), where q is...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|
- Enthalpy of neutralzation is defined as the enthalpy change when 1 mol...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Match the following
Text Solution
|
- Match the following:
Text Solution
|
- Match the following Columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- The feasibility of a chemical reaction can be explained based on DH, D...
Text Solution
|
- Match the following :
Text Solution
|
- Statement-1: For a process to be spontaneous, Delta G as well as Delta...
Text Solution
|
- Assertion:Phase transition involves change in internal energy only. ...
Text Solution
|