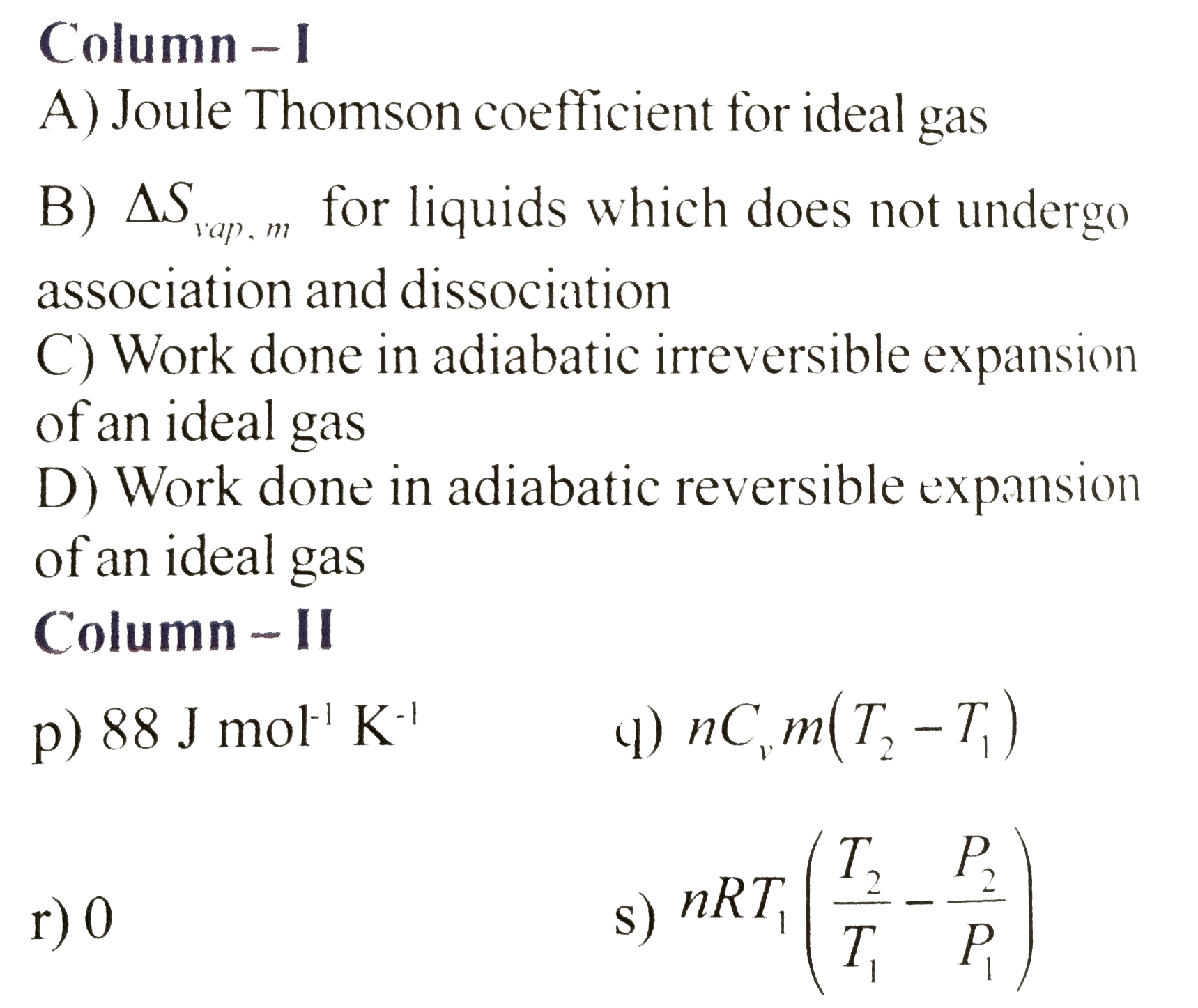Text Solution
Verified by Experts
The correct Answer is:
NARAYNA-THERMODYNAMICS-Level -V
- Work done by the system in isothermal reversible process is w(rev.)= -...
Text Solution
|
- Match the following
Text Solution
|
- Match the following:
Text Solution
|
- Match the following Columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- The feasibility of a chemical reaction can be explained based on DH, D...
Text Solution
|
- Match the following :
Text Solution
|
- Statement-1: For a process to be spontaneous, Delta G as well as Delta...
Text Solution
|
- Assertion:Phase transition involves change in internal energy only. ...
Text Solution
|
- Statement-1 : The work done in an open container at 300 K, when 112 g ...
Text Solution
|
- Statement-1 : Many endothermic reactions that are not spontaneous at r...
Text Solution
|
- Statement: In the case of an ideal gas the changes in Gibbs and Helmho...
Text Solution
|
- STATEMENT-1 : The Heat absorbed during the isothermal expansionof an i...
Text Solution
|
- Each question contains STATEMENT-1 (Assertion) and STATEMENT-2( Reason...
Text Solution
|
- Assertion: C(P)-C(V)=R for an ideal gas. Reason: ((delE)/(delV))(T)=...
Text Solution
|
- Statement-1 : The amount of heat change during the isothermal free exp...
Text Solution
|
- Statement-1: The enthalpy of formation of H(2)O(l)is greater than of H...
Text Solution
|
- Statement-1: Heat of neutralisation of H(3)PO(4) with NaOH is more tha...
Text Solution
|
- Statement-1 : Work done in isothermal reversible process is more than ...
Text Solution
|