Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-THERMODYNAMICS-Level -V
- Staetement -1: The magniyude of the work involed in an isothermal expa...
Text Solution
|
- Statement -1: For every chmical reaction at equilibrium , standard Gid...
Text Solution
|
- Statement-1: There is a natural asymmetry between work to heat and con...
Text Solution
|
- Statement -1: Entropy change in reversible adiabatic expansion of an i...
Text Solution
|
- Statement -1: The Standard free energy changes of all spontaneously oc...
Text Solution
|
- Statement -1: Enthalpy and entropy of any elements substance in the st...
Text Solution
|
- Statement-1: A reaction which is spontaneous and accompained by decrea...
Text Solution
|
- Statement -1: Many endothermic reactions that are not spontaneous at r...
Text Solution
|
- Statement-1: Decrease of free energy during the process under constant...
Text Solution
|
- Statement-1: All combustion reactions are exothermic. Statement-2: E...
Text Solution
|
- Statement-1: Due to adiabatic free expansion temperature of real gas m...
Text Solution
|
- Statement-1: Under adiabatic free expansion, ((dU)/(dV))(T) is +ve whe...
Text Solution
|
- Statement-1: At low temperatures, DH is the dominant factor for sponta...
Text Solution
|
- Statement-1: A reaction which is spontaneous and accompained by decrea...
Text Solution
|
- The temperature of 1 mole helium gas is increased by 1^@C. Find the in...
Text Solution
|
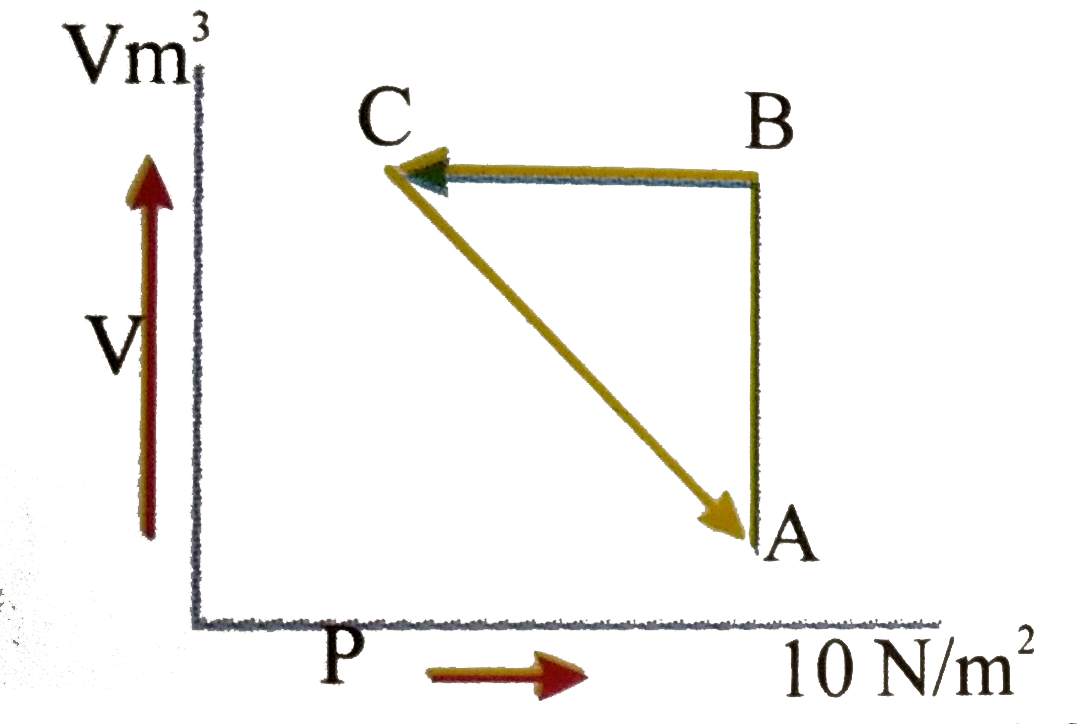
- An ideal gas is taken through the cycle A rarr B rarr C rarr A As show...
Text Solution
|
- If 2kcal heat is given to a system and 6 kcal work is done on the syst...
Text Solution
|
- The constant volume molar heat capacity of an ideal gas is expressed b...
Text Solution
|
- Molar enthalpy of vaporization of a liquid is 2.6 kJ. If boiling point...
Text Solution
|
- The chemical reaction : A rarr P, Delta H^(@) = 2.8 kJ is spontaneous ...
Text Solution
|