A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BITSAT GUIDE-ALCOHOLS, PHENOLS AND ETHERS-BITSAT Archives
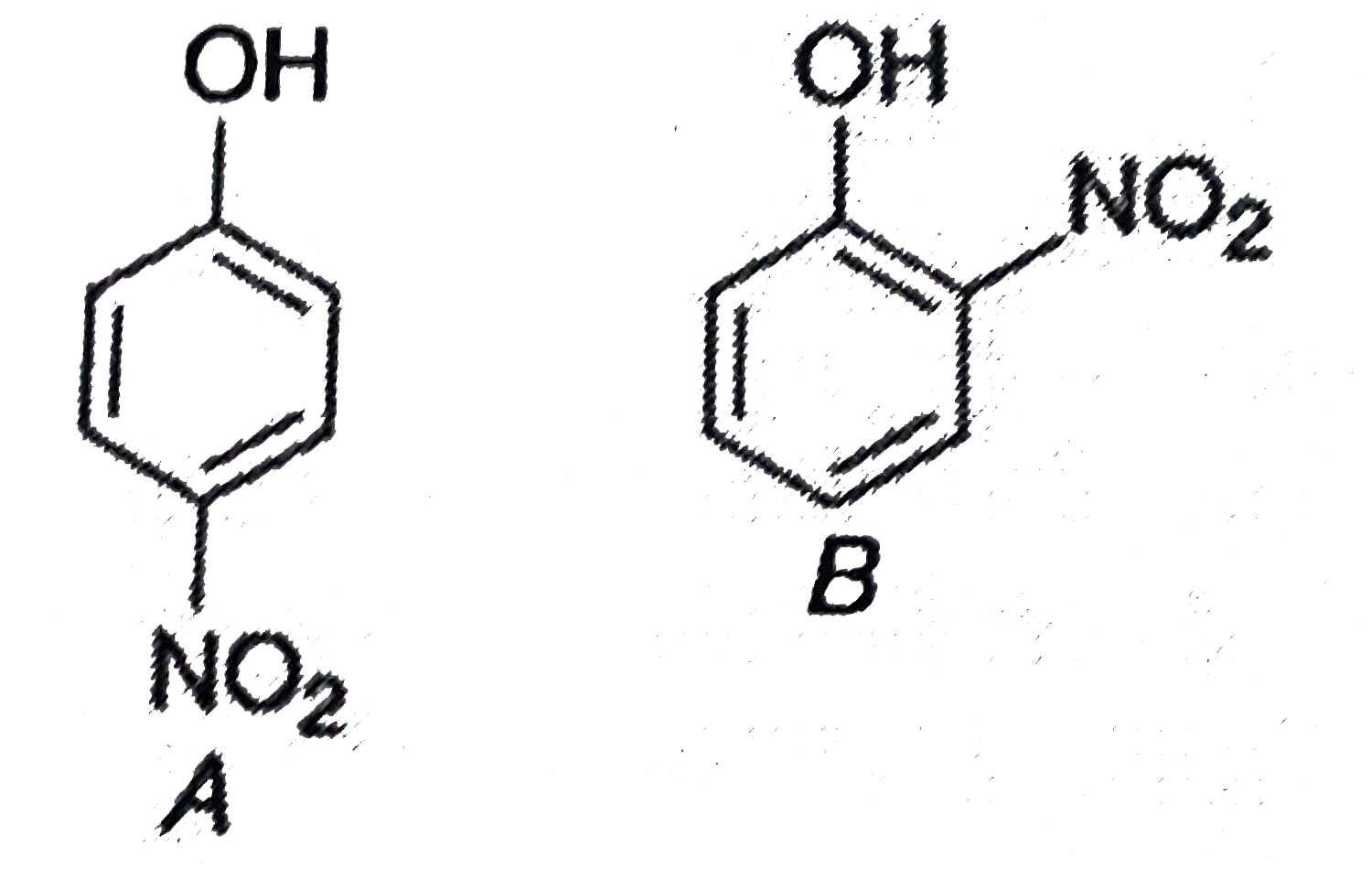
- Consider the following compounds Among the compounds A and B, the...
Text Solution
|
- What will be the product of the following reaction ? {:(" "...
Text Solution
|
- Identify the correct prouct formed during the following reaction :
Text Solution
|
- When 2-methyl propan-2-ol is treated with a mixture of conc. HCl and Z...
Text Solution
|
- What will be the correct relation between products when 2-methyl cyclo...
Text Solution
|
- The following reaction is known as
Text Solution
|
- The reaction is called
Text Solution
|
- 3-methyl-2-butanol on treatment with HCl gives (major product)
Text Solution
|
- Phenol can be tested by
Text Solution
|
- Phenol react with PCl(5) to give mainly
Text Solution
|
- The IUPAC name of
Text Solution
|
- The compound which gives the most stable carbonium ion on dehydration ...
Text Solution
|
- Pinacol is
Text Solution
|
- Grignard reagent reacts with HCHO to produce a/an
Text Solution
|
- The product obtained by heating diethyl ether with HI is
Text Solution
|