A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA- ELECTRO CHEMISTRY-LEVEL-I(H.W)
- The reason for increase in electrical conduction of a weak electrolyte...
Text Solution
|
- The specific conductances of four electrolytes in ohm^(-1)cm^(-1) are ...
Text Solution
|
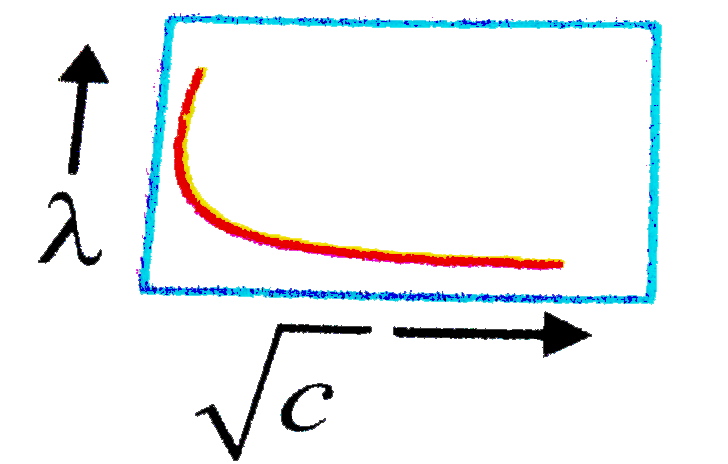
- The variation of equivalent conductance of a weak electrolyte with (co...
Text Solution
|
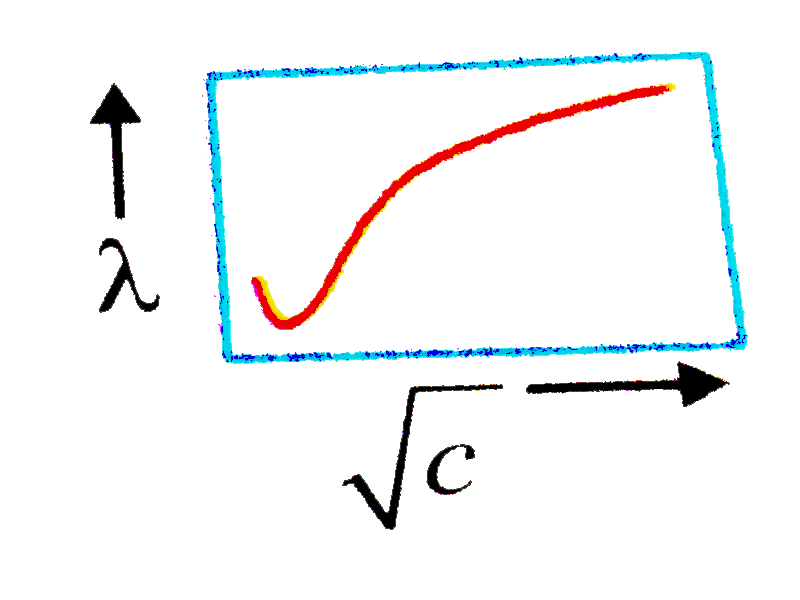
- The variation equivalent conductance of stronge electrolyte with sqrt(...
Text Solution
|
- Equivalent conductance of 1M CH(3)COOH is 10ohm^(-1) cm^(2) "equiv"^(-...
Text Solution
|
- if ^^(c) of NH(4)OH is 11.5Omega^(-1) cm^(2) mol^(-1), its degree of d...
Text Solution
|
- The values of wedge(m)^(oo) forNH(4)Cl,NaOH, and NaCl are, respectivel...
Text Solution
|
- The molar conductances of NaCI, HCI and CH3 COONa at infinite dilution...
Text Solution
|
- If a salt bridge is not used between two half cells, voltage
Text Solution
|
- An electrochemical cell stops working after some time because
Text Solution
|
- Consider a voltaic cell based on these half - cell reactions Ag^(+)(...
Text Solution
|
- A reversible galvanic cell is connected to an external battery. If the...
Text Solution
|
- The EMF of the cell Ni|Ni^(2+) (0.01M) Cl^(-)(0.01M)//Cl(2), pt is ...
Text Solution
|
- The reduction potential of a hydrogen electrode at pH 10 at 298K is : ...
Text Solution
|
- The oxidation potential of 0.05 MH(2)SO(4) is
Text Solution
|
- Number of Faradays involved in the net reaction of Lead accumulator is
Text Solution
|
- Following are some of the facts about a dry cell I : It is alos call...
Text Solution
|
- Iron rod is dipped in concentrated HNO(3) After some time the iron rod...
Text Solution
|
- Which of the following is correct regarding mechanical passivity
Text Solution
|
- Zn acts as sacrifical or cathodic protect iont to prevent rusting of i...
Text Solution
|