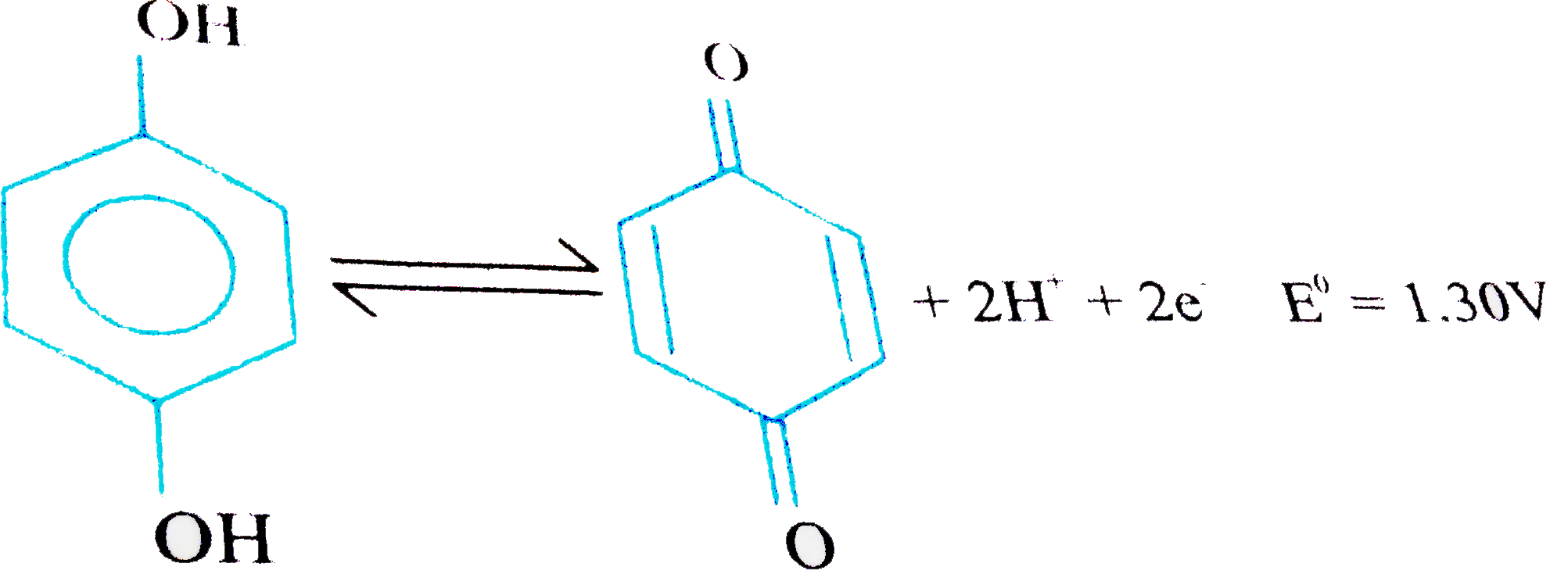
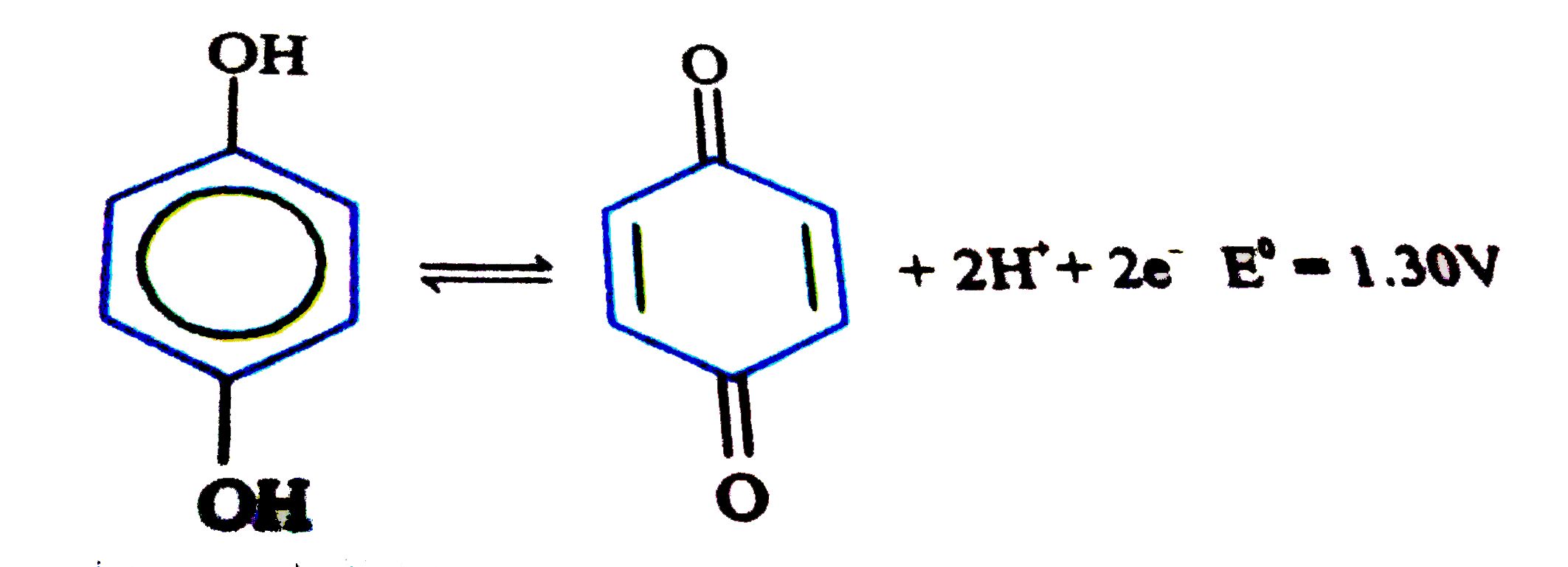A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA- ELECTRO CHEMISTRY-LEVEL-VI
- In two vessels each containing 500mL water, 0.05m mol of aniline (K(b)...
Text Solution
|
- Construct the cell corresponding to the reaction : 3Cr^(2+)(1M)rarr 2C...
Text Solution
|
- For the half cell At pH = 3 electrode potential is
Text Solution
|
- For the galvanic cell : Ag|AgCl(s)),KCl(0.2M)||KBr(0.001M),AgBr(s)|A...
Text Solution
|
- Consider the reaction fo extraction of gold from its ore Au +2CN^(-)...
Text Solution
|
- A hydrogen electrode X was placed in a buffer solution of sodium aceta...
Text Solution
|
- An saturated solution in AgA (K(sp) = 3 xx 10^(-14)) and AgB(K(sp) = 1...
Text Solution
|
- The standard emf of the cell. Cd(s) | CdCl(2) (aq) rightarrow 2Ag(s)...
Text Solution
|
- The standard electrode potentials, E(I(2)//I^(-))^(@), E(Br^(-)//Br(2)...
Text Solution
|
- In which of the following (E("cell")-E("cell")^(@))=0
Text Solution
|
- Select the correct statements if 9.65 A current is passed for 1 hour t...
Text Solution
|
- The electolysis of acetate solution produces ethane according to react...
Text Solution
|
- 100ml of 0.05M CuSO(4)("aq") solution was electrolysed using inert ele...
Text Solution
|
- What is the potential of an electrode which originally contained 0.1 M...
Text Solution
|
- E^(@) in the given diagram is
Text Solution
|
- Cr(2)O(7)^(-2)+14H^(+)+6Fe^(+2)rarr2Cr^(+3)+6Fe^(+3)+7H(2)O Given : ...
Text Solution
|
- During the working of the following cell Pb-Hg(1M)|Pb^(2+)(aq)(0.1M)...
Text Solution
|
- A standard solution of KNO(3) is used to make salt bridge, because
Text Solution
|
- For the calomel half-cell, Hg, Hg(2)Cl(2)|Cl^(-)(aq) values of electro...
Text Solution
|
- At what [Br^(-)]/sqrt(CO(3)^(2-)] does the following cell have its rea...
Text Solution
|