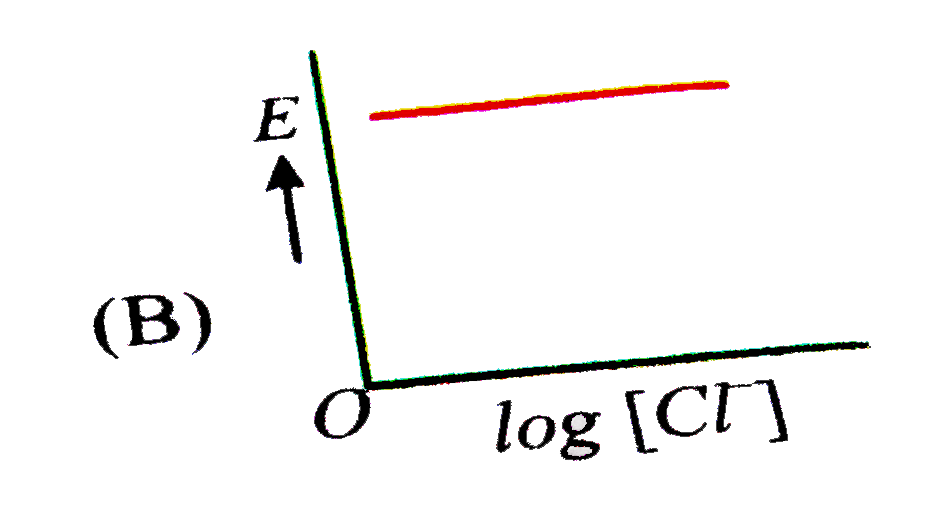
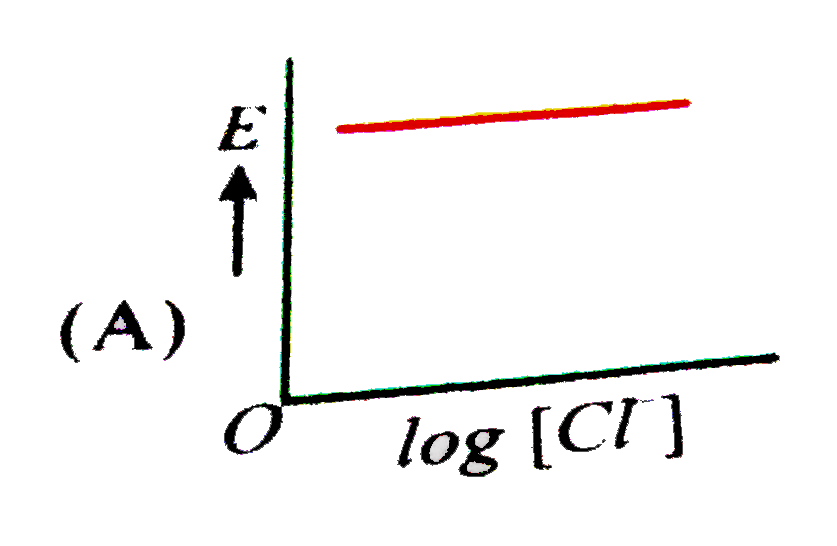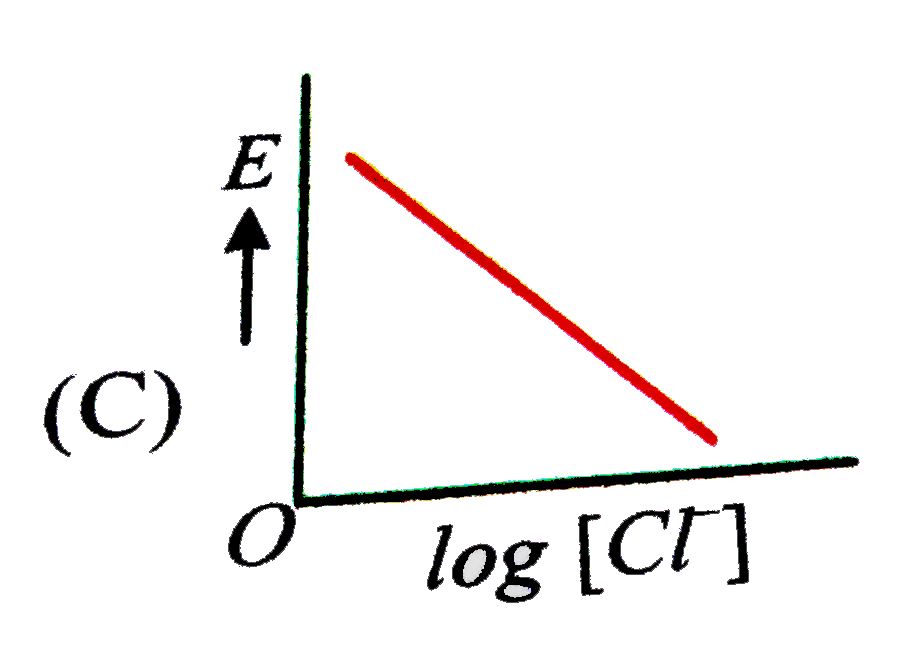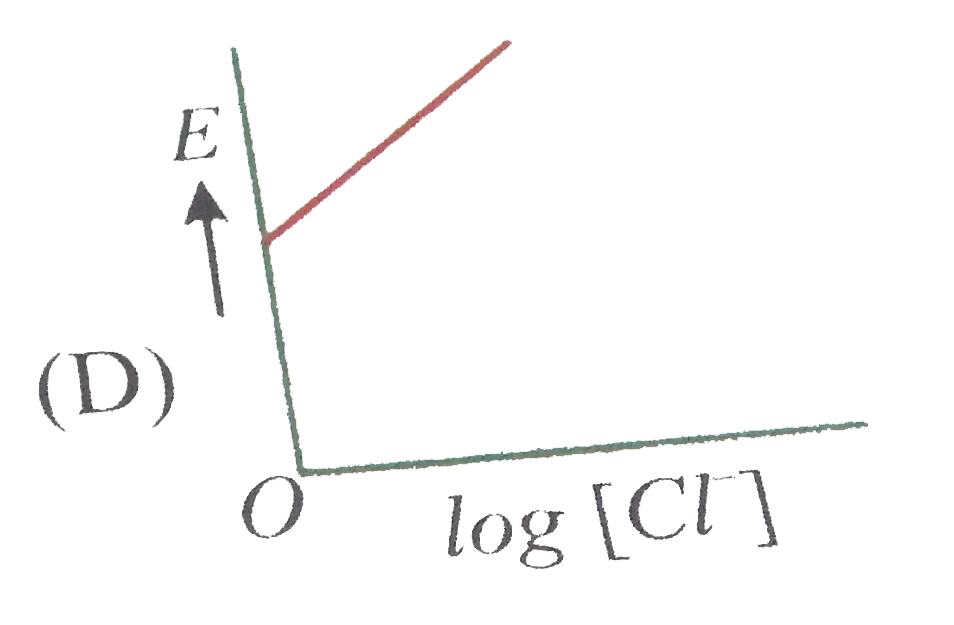A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA- ELECTRO CHEMISTRY-LEVEL-VI
- During the working of the following cell Pb-Hg(1M)|Pb^(2+)(aq)(0.1M)...
Text Solution
|
- A standard solution of KNO(3) is used to make salt bridge, because
Text Solution
|
- For the calomel half-cell, Hg, Hg(2)Cl(2)|Cl^(-)(aq) values of electro...
Text Solution
|
- At what [Br^(-)]/sqrt(CO(3)^(2-)] does the following cell have its rea...
Text Solution
|
- Calcualte the EMF of the cell at 298K Pt|H(2)(1atm) | NaOH(xM), Na...
Text Solution
|
- When a galvanic cell starts operating, with passage of time,
Text Solution
|
- Rusting on the surface of iron involves :
Text Solution
|
- Select the correct statement(s) regarding refrence electrodes
Text Solution
|
- Indicate the correct statements :
Text Solution
|
- Which of the following statements is/are correct ?
Text Solution
|
- When a concentrated solution of an electrolyte is diluted
Text Solution
|
- Given that E(Ni^(2+)//Ni)^(@)=-0.25V, E(Cu^(2+)//Cu)^(@)=+0.34V, E...
Text Solution
|
- Which of the following statements is correct? If E(Cu^(2+)|Cu)^(@)=0.3...
Text Solution
|
- 2 amperes of current is passed for 16 min and 5 second through 1000 ml...
Text Solution
|
- For the cell Tl|Tl^(+)(0.001M)||Cu^(2+)(0.01M)|Cu.E("cell") at 25^(@)C...
Text Solution
|
- For the cell (at 298K) Ag(s) | AgCl(s) | Cl^(-)(aq)|| AgNO(3)(aq) | ...
Text Solution
|
- The potential of an electrode when each species involved in it exists ...
Text Solution
|
- The potential of an electrode when each species involved in it exists ...
Text Solution
|
- The potential of an electrode when each species involved in it exists ...
Text Solution
|
- In a lead storag battery, Pb (anode) and PbO(2) (cathode) are used. Co...
Text Solution
|